N-3 polyunsaturated fatty acids block the trimethylamine-N-oxide- ACE2- TMPRSS2 cascade to inhibit the infection of human endothelial progenitor cells by SARS-CoV-2
- PMID: 35817244
- PMCID: PMC9264727
- DOI: 10.1016/j.jnutbio.2022.109102
N-3 polyunsaturated fatty acids block the trimethylamine-N-oxide- ACE2- TMPRSS2 cascade to inhibit the infection of human endothelial progenitor cells by SARS-CoV-2
Abstract
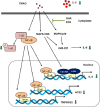
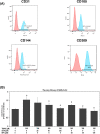
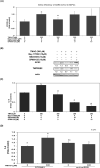
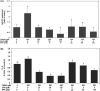
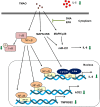
Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) is a novel coronavirus that infects many types of cells and causes cytokine storms, excessive inflammation, acute respiratory distress to induce failure of respiratory system and other critical organs. In this study, our results showed that trimethylamine-N-oxide (TMAO), a metabolite generated by gut microbiota, acts as a regulatory mediator to enhance the inerleukin-6 (IL-6) cytokine production and the infection of human endothelial progenitor cells (hEPCs) by SARS-CoV-2. Treatment of N-3 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) could effectively block the entry of SARS-CoV-2 in hEPCs. The anti-infection effects of N-3 PUFAs were associated with the inactivation of NF-κB signaling pathway, a decreased expression of the entry receptor angiotensin-converting enzyme 2 (ACE2) and downstream transmembrane serine protease 2 in hEPCs upon the stimulation of TMAO. Treatment of DHA and EPA further effectively inhibited TMAO-mediated expression of IL-6 protein, probably through an inactivation of MAPK/p38/JNK signaling cascades and a downregulation of microRNA (miR)-221 in hEPCs. In conclusion, N-3 PUFAs such as DHA and EPA could effectively act as preventive agents to block the infection of SARS-CoV-2 and IL-6 cytokine production in hEPCs upon the stimulation of TMAO.
Keywords: SARS-CoV-2; Trimethylamine-N-oxide; human endothelial progenitor cells; interleukin-6; microRNA-221.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Docosahexaenoic Acid Alleviates Trimethylamine-N-oxide-mediated Impairment of Neovascularization in Human Endothelial Progenitor Cells.Nutrients. 2023 May 4;15(9):2190. doi: 10.3390/nu15092190. Nutrients. 2023. PMID: 37432325 Free PMC article.
-
Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry.Int J Mol Sci. 2022 Nov 10;23(22):13825. doi: 10.3390/ijms232213825. Int J Mol Sci. 2022. PMID: 36430303 Free PMC article.
-
Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators.Redox Biol. 2020 Sep;36:101615. doi: 10.1016/j.redox.2020.101615. Epub 2020 Jun 24. Redox Biol. 2020. PMID: 32863223 Free PMC article.
-
Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review.Int J Mol Sci. 2021 Apr 26;22(9):4526. doi: 10.3390/ijms22094526. Int J Mol Sci. 2021. PMID: 33926110 Free PMC article. Review.
-
Interaction of SARS-CoV-2 and Other Coronavirus With ACE (Angiotensin-Converting Enzyme)-2 as Their Main Receptor: Therapeutic Implications.Hypertension. 2020 Nov;76(5):1339-1349. doi: 10.1161/HYPERTENSIONAHA.120.15256. Epub 2020 Aug 27. Hypertension. 2020. PMID: 32851855 Free PMC article. Review.
Cited by
-
Gut Microbiota and COVID-19: Potential Implications for Disease Severity.Pathogens. 2022 Sep 15;11(9):1050. doi: 10.3390/pathogens11091050. Pathogens. 2022. PMID: 36145482 Free PMC article. Review.
-
Nutrition at the Intersection between Gut Microbiota Eubiosis and Effective Management of Type 2 Diabetes.Nutrients. 2024 Jan 16;16(2):269. doi: 10.3390/nu16020269. Nutrients. 2024. PMID: 38257161 Free PMC article. Review.
-
Research progress of diabetic retinopathy and gut microecology.Front Microbiol. 2023 Sep 7;14:1256878. doi: 10.3389/fmicb.2023.1256878. eCollection 2023. Front Microbiol. 2023. PMID: 37744925 Free PMC article. Review.
-
Docosahexaenoic Acid Alleviates Trimethylamine-N-oxide-mediated Impairment of Neovascularization in Human Endothelial Progenitor Cells.Nutrients. 2023 May 4;15(9):2190. doi: 10.3390/nu15092190. Nutrients. 2023. PMID: 37432325 Free PMC article.
-
Regulation of SARS-CoV-2 infection by diet-modulated gut microbiota.Front Cell Infect Microbiol. 2023 Jun 29;13:1167827. doi: 10.3389/fcimb.2023.1167827. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37457959 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

