Contexts and contradictions: a roadmap for computational drug repurposing with knowledge inference
- PMID: 35817308
- PMCID: PMC9294417
- DOI: 10.1093/bib/bbac268
Contexts and contradictions: a roadmap for computational drug repurposing with knowledge inference
Abstract
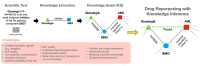
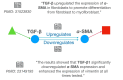
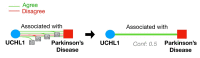
The cost of drug development continues to rise and may be prohibitive in cases of unmet clinical need, particularly for rare diseases. Artificial intelligence-based methods are promising in their potential to discover new treatment options. The task of drug repurposing hypothesis generation is well-posed as a link prediction problem in a knowledge graph (KG) of interacting of drugs, proteins, genes and disease phenotypes. KGs derived from biomedical literature are semantically rich and up-to-date representations of scientific knowledge. Inference methods on scientific KGs can be confounded by unspecified contexts and contradictions. Extracting context enables incorporation of relevant pharmacokinetic and pharmacodynamic detail, such as tissue specificity of interactions. Contradictions in biomedical KGs may arise when contexts are omitted or due to contradicting research claims. In this review, we describe challenges to creating literature-scale representations of pharmacological knowledge and survey current approaches toward incorporating context and resolving contradictions.
Keywords: drug repurposing; knowledge graphs; metascience; natural language processing.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Similar articles
-
Knowledge Graphs for drug repurposing: a review of databases and methods.Brief Bioinform. 2024 Sep 23;25(6):bbae461. doi: 10.1093/bib/bbae461. Brief Bioinform. 2024. PMID: 39325460 Free PMC article. Review.
-
Protocol to implement a computational pipeline for biomedical discovery based on a biomedical knowledge graph.STAR Protoc. 2023 Dec 15;4(4):102666. doi: 10.1016/j.xpro.2023.102666. Epub 2023 Oct 25. STAR Protoc. 2023. PMID: 37883224 Free PMC article.
-
Explainable drug repurposing via path based knowledge graph completion.Sci Rep. 2024 Jul 18;14(1):16587. doi: 10.1038/s41598-024-67163-x. Sci Rep. 2024. PMID: 39025897 Free PMC article.
-
MPTN: A message-passing transformer network for drug repurposing from knowledge graph.Comput Biol Med. 2024 Jan;168:107800. doi: 10.1016/j.compbiomed.2023.107800. Epub 2023 Dec 1. Comput Biol Med. 2024. PMID: 38043469
-
Application of artificial intelligence and machine learning in drug repurposing.Prog Mol Biol Transl Sci. 2024;205:171-211. doi: 10.1016/bs.pmbts.2024.03.030. Epub 2024 Mar 31. Prog Mol Biol Transl Sci. 2024. PMID: 38789178 Review.
Cited by
-
Editorial: Emerging areas in literature-based discovery.Front Res Metr Anal. 2023 Jan 19;8:1122547. doi: 10.3389/frma.2023.1122547. eCollection 2023. Front Res Metr Anal. 2023. PMID: 36741345 Free PMC article. No abstract available.
-
Developing a Knowledge Graph for Pharmacokinetic Natural Product-Drug Interactions.J Biomed Inform. 2023 Apr;140:104341. doi: 10.1016/j.jbi.2023.104341. Epub 2023 Mar 17. J Biomed Inform. 2023. PMID: 36933632 Free PMC article.
-
Knowledge Graphs for drug repurposing: a review of databases and methods.Brief Bioinform. 2024 Sep 23;25(6):bbae461. doi: 10.1093/bib/bbae461. Brief Bioinform. 2024. PMID: 39325460 Free PMC article. Review.
-
Discovering causal paths to diabetic nephropathy by combining computable biomedical knowledge with graph mining algorithms.AMIA Annu Symp Proc. 2023 Apr 29;2022:1118-1124. eCollection 2022. AMIA Annu Symp Proc. 2023. PMID: 37128414 Free PMC article.
-
Modeling Path Importance for Effective Alzheimer's Disease Drug Repurposing.Pac Symp Biocomput. 2024;29:306-321. Pac Symp Biocomput. 2024. PMID: 38160288 Free PMC article.
References
-
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016;47:20–33. - PubMed
-
- Kantarjian HM, Prat F, Steensma DP, et al. Cancer research in the United States: a critical review of current status and proposal for alternative models. Cancer 2018;124(14):2881–9. - PubMed
-
- Milsted RAV. Cancer drug approval in the United States, Europe, and Japan. Adv Cancer Res 2006;96:371–91. - PubMed
-
- Harrison RK. Phase ii and phase iii failures: 2013-2015. Nat Rev Drug Discov 2016;15(12):817–8. - PubMed
-
- Doench JG. Am I ready for CRISPR? A user’s guide to genetic screens. Nat Rev Genet 2018;19(2):67–80. - PubMed

