Cellular Senescence in Acute and Chronic Wound Repair
- PMID: 35817510
- PMCID: PMC9620855
- DOI: 10.1101/cshperspect.a041221
Cellular Senescence in Acute and Chronic Wound Repair
Abstract
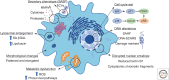
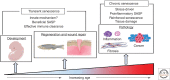
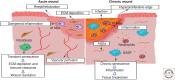
Cellular senescence, once thought an artifact of in vitro culture or passive outcome of aging, has emerged as fundamental to tissue development and function. The senescence mechanism importantly halts cell cycle progression to protect against tumor formation, while transiently present senescent cells produce a complex secretome (or SASP) of inflammatory mediators, proteases, and growth factors that guide developmental remodeling and tissue regeneration. Transiently present senescence is important for skin repair, where it accelerates extracellular matrix formation, limits fibrosis, promotes reepithelialization, and modulates inflammation. Unfortunately, advanced age and diabetes drive pathological accumulation of senescent cells in chronic wounds, which is perpetuated by a proinflammatory SASP, advanced glycation end-products, and oxidative damage. Although the biology of wound senescence remains incompletely understood, drugs that selectively target senescent cells are showing promise in clinical trials for diverse pathological conditions. It may not be long before senescence-targeted therapies will be available for the management, or perhaps even prevention, of chronic wounds.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Targeting Senescent Cells: Possible Implications for Delaying Skin Aging: A Mini-Review.Gerontology. 2016;62(5):513-8. doi: 10.1159/000444877. Epub 2016 Apr 1. Gerontology. 2016. PMID: 27031122 Review.
-
Cellular senescence in bone.Bone. 2019 Apr;121:121-133. doi: 10.1016/j.bone.2019.01.015. Epub 2019 Jan 16. Bone. 2019. PMID: 30659978 Free PMC article. Review.
-
Modelling the spatiotemporal dynamics of senescent cells in wound healing, chronic wounds, and fibrosis.PLoS Comput Biol. 2025 Apr 15;21(4):e1012298. doi: 10.1371/journal.pcbi.1012298. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40233102 Free PMC article.
-
Cellular senescence with SASP in periodontal ligament cells triggers inflammation in aging periodontal tissue.Aging (Albany NY). 2023 Mar 1;15(5):1279-1305. doi: 10.18632/aging.204569. Epub 2023 Mar 1. Aging (Albany NY). 2023. PMID: 36863315 Free PMC article.
-
Potential Regulators of the Senescence-Associated Secretory Phenotype During Senescence and Aging.J Gerontol A Biol Sci Med Sci. 2022 Nov 21;77(11):2207-2218. doi: 10.1093/gerona/glac097. J Gerontol A Biol Sci Med Sci. 2022. PMID: 35524726 Review.
Cited by
-
The Skin Microbiome: Current Landscape and Future Opportunities.Int J Mol Sci. 2023 Feb 16;24(4):3950. doi: 10.3390/ijms24043950. Int J Mol Sci. 2023. PMID: 36835363 Free PMC article. Review.
-
The interplay of cellular senescence and reprogramming shapes the biological landscape of aging and cancer revealing novel therapeutic avenues.Front Cell Dev Biol. 2025 Apr 28;13:1593096. doi: 10.3389/fcell.2025.1593096. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40356604 Free PMC article. Review.
-
Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review.Pharmaceuticals (Basel). 2025 May 24;18(6):787. doi: 10.3390/ph18060787. Pharmaceuticals (Basel). 2025. PMID: 40573181 Free PMC article. Review.
-
Senescence and Stress Signaling Pathways in Corneal Cells After Nitrogen Mustard Injury.Cells. 2024 Dec 6;13(23):2021. doi: 10.3390/cells13232021. Cells. 2024. PMID: 39682768 Free PMC article.
-
Glucose oxidase: An emerging multidimensional treatment option for diabetic wound healing.Bioact Mater. 2024 Oct 15;44:131-151. doi: 10.1016/j.bioactmat.2024.10.006. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39484022 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
