Extracellular Matrix Regulation of Vascular Morphogenesis, Maturation, and Stabilization
- PMID: 35817544
- PMCID: PMC10578078
- DOI: 10.1101/cshperspect.a041156
Extracellular Matrix Regulation of Vascular Morphogenesis, Maturation, and Stabilization
Abstract
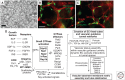
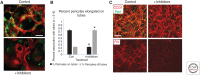
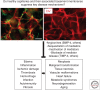
The extracellular matrix represents a critical regulator of tissue vascularization during embryonic development and postnatal life. In this perspective, we present key information and concepts that focus on how the extracellular matrix controls capillary assembly, maturation, and stabilization, and, in addition, contributes to tissue stability and health. In particular, we present and discuss mechanistic details underlying (1) the role of the extracellular matrix in controlling different steps of vascular morphogenesis, (2) the ability of endothelial cells (ECs) and pericytes to coassemble into elongated and narrow capillary EC-lined tubes with associated pericytes and basement membrane matrices, and (3) the identification of specific growth factor combinations ("factors") and peptides as well as coordinated "factor" and extracellular matrix receptor signaling pathways that are required to form stabilized capillary networks.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
