Inter-Animal Variability in Activity Phase Is Constrained by Synaptic Dynamics in an Oscillatory Network
- PMID: 35817566
- PMCID: PMC9319424
- DOI: 10.1523/ENEURO.0027-22.2022
Inter-Animal Variability in Activity Phase Is Constrained by Synaptic Dynamics in an Oscillatory Network
Abstract
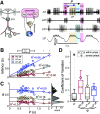
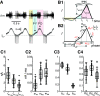
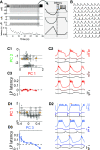
The levels of voltage-gated and synaptic currents in the same neuron type can vary substantially across individuals. Yet, the phase relationships between neurons in oscillatory circuits are often maintained, even in the face of varying oscillation frequencies. We examined whether synaptic and intrinsic currents are matched to maintain constant activity phases across preparations, using the lateral pyloric (LP) neuron of the stomatogastric ganglion (STG) of the crab, Cancer borealis LP produces stable oscillatory bursts on release from inhibition, with an onset phase that is independent of oscillation frequency. We quantified the parameters that define the shape of the synaptic current inputs across preparations and found no linear correlations with voltage-gated currents. However, several synaptic parameters were correlated with oscillation period and burst onset phase, suggesting they may play a role in phase maintenance. We used dynamic clamp to apply artificial synaptic inputs and found that those synaptic parameters correlated with phase and period were ineffective in influencing burst onset. Instead, parameters that showed the least variability across preparations had the greatest influence. Thus, parameters that influence circuit phasing are constrained across individuals, while those that have little effect simply co-vary with phase and frequency.
Keywords: correlations; dynamic clamp; oscillation; phase maintenance; stomatogastric; synaptic dynamics.
Copyright © 2022 Anwar et al.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
