Natural History of Friedreich Ataxia: Heterogeneity of Neurologic Progression and Consequences for Clinical Trial Design
- PMID: 35817567
- PMCID: PMC9576299
- DOI: 10.1212/WNL.0000000000200913
Natural History of Friedreich Ataxia: Heterogeneity of Neurologic Progression and Consequences for Clinical Trial Design
Abstract
Background and objectives: The understanding of the natural history of Friedreich ataxia (FRDA) has improved considerably recently, but patterns of neurologic deterioration are not fully clarified, compromising the assessment of the clinical relevance of effects and guidance for study design. The goal of this study was to acknowledge the broad genetic diversity of the population, especially for younger individuals, and to provide analyses stratified by age to guide population selection in future studies.
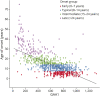
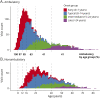
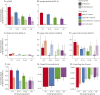
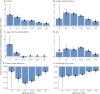
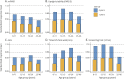
Methods: Based on a large natural history study, the FRDA Clinical Outcome Measures study that at the current data cut enrolled 1,115 participants, followed up for 5,287 yearly visits, we present results from the modified FRDA Rating Scale and its subscores. The secondary outcomes included the patient-reported activities of daily living scale, the timed 25-foot walk, and the 9-hole peg test. Long-term progression was modeled using slope analyses within early-onset, typical-onset, intermediate-onset, and late-onset FRDA. To reflect recruitment in clinical trials, short-term changes were analyzed within age-based subpopulations. All analyses were stratified by ambulation status.
Results: Long-term progression models stratified by disease severity indicated highly differential disease progression, especially at earlier ages at onset. In the ambulatory phase, decline was driven by axial items assessed by the Upright Stability subscore of the mFARS. The analyses of short-term changes showed slower progression with increasing population age due to decreasing genetic severity. Future clinical studies could reduce population diversity, interpatient variability, and the risk of imbalanced treatment groups by selecting the study population based on the functional capacity (e.g., ambulatory status) and by strict age-based stratification.
Discussion: The understanding of the diversity within FRDA populations and their patterns of functional decline provides an essential foundation for future clinical trial design including patient selection and facilitates the interpretation of the clinical relevance of progression detected in FRDA.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures