Cell fate decisions, transcription factors and signaling during early retinal development
- PMID: 35817658
- PMCID: PMC9669153
- DOI: 10.1016/j.preteyeres.2022.101093
Cell fate decisions, transcription factors and signaling during early retinal development
Abstract
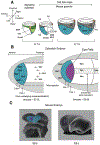
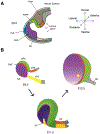
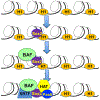
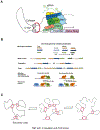
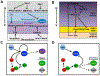
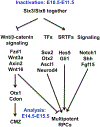
The development of the vertebrate eyes is a complex process starting from anterior-posterior and dorso-ventral patterning of the anterior neural tube, resulting in the formation of the eye field. Symmetrical separation of the eye field at the anterior neural plate is followed by two symmetrical evaginations to generate a pair of optic vesicles. Next, reciprocal invagination of the optic vesicles with surface ectoderm-derived lens placodes generates double-layered optic cups. The inner and outer layers of the optic cups develop into the neural retina and retinal pigment epithelium (RPE), respectively. In vitro produced retinal tissues, called retinal organoids, are formed from human pluripotent stem cells, mimicking major steps of retinal differentiation in vivo. This review article summarizes recent progress in our understanding of early eye development, focusing on the formation the eye field, optic vesicles, and early optic cups. Recent single-cell transcriptomic studies are integrated with classical in vivo genetic and functional studies to uncover a range of cellular mechanisms underlying early eye development. The functions of signal transduction pathways and lineage-specific DNA-binding transcription factors are dissected to explain cell-specific regulatory mechanisms underlying cell fate determination during early eye development. The functions of homeodomain (HD) transcription factors Otx2, Pax6, Lhx2, Six3 and Six6, which are required for early eye development, are discussed in detail. Comprehensive understanding of the mechanisms of early eye development provides insight into the molecular and cellular basis of developmental ocular anomalies, such as optic cup coloboma. Lastly, modeling human development and inherited retinal diseases using stem cell-derived retinal organoids generates opportunities to discover novel therapies for retinal diseases.
Keywords: Cell determination; Ciliary marginal zone; Differentiation; Ectoderm; Homeodomain; Lhx2; Neuroectoderm; Optic cup; Otx2; Pax6; Retinal pigmented epithelium; Retinal progenitor cells; Six3; Six6.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brûlet P, 1995. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121, 3279–3290. - PubMed
-
- Adler R, Belecky-Adams TL, 2002. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development 129, 3161–3171. - PubMed
-
- Agoston Z, Schulte D, 2009. Meis2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrainhindbrain boundary organizer. Development 136, 3311–3322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

