Reduced neural feedback signaling despite robust neuron and gamma auditory responses during human sleep
- PMID: 35817847
- PMCID: PMC9276533
- DOI: 10.1038/s41593-022-01107-4
Reduced neural feedback signaling despite robust neuron and gamma auditory responses during human sleep
Erratum in
-
Author Correction: Reduced neural feedback signaling despite robust neuron and gamma auditory responses during human sleep.Nat Neurosci. 2022 Aug;25(8):1113. doi: 10.1038/s41593-022-01137-y. Nat Neurosci. 2022. PMID: 35853999 Free PMC article. No abstract available.
Abstract
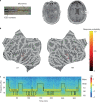
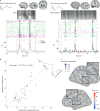
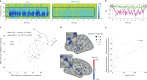
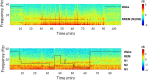
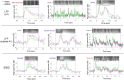
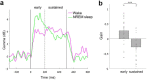
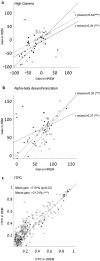
During sleep, sensory stimuli rarely trigger a behavioral response or conscious perception. However, it remains unclear whether sleep inhibits specific aspects of sensory processing, such as feedforward or feedback signaling. Here, we presented auditory stimuli (for example, click-trains, words, music) during wakefulness and sleep in patients with epilepsy, while recording neuronal spiking, microwire local field potentials, intracranial electroencephalogram and polysomnography. Auditory stimuli induced robust and selective spiking and high-gamma (80-200 Hz) power responses across the lateral temporal lobe during both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Sleep only moderately attenuated response magnitudes, mainly affecting late responses beyond early auditory cortex and entrainment to rapid click-trains in NREM sleep. By contrast, auditory-induced alpha-beta (10-30 Hz) desynchronization (that is, decreased power), prevalent in wakefulness, was strongly reduced in sleep. Thus, extensive auditory responses persist during sleep whereas alpha-beta power decrease, likely reflecting neural feedback processes, is deficient. More broadly, our findings suggest that feedback signaling is key to conscious sensory processing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures