A nanoparticle probe for the imaging of autophagic flux in live mice via magnetic resonance and near-infrared fluorescence
- PMID: 35817962
- PMCID: PMC9492651
- DOI: 10.1038/s41551-022-00904-3
A nanoparticle probe for the imaging of autophagic flux in live mice via magnetic resonance and near-infrared fluorescence
Abstract
Autophagy-the lysosomal degradation of cytoplasmic components via their sequestration into double-membraned autophagosomes-has not been detected non-invasively. Here we show that the flux of autophagosomes can be measured via magnetic resonance imaging or serial near-infrared fluorescence imaging of intravenously injected iron oxide nanoparticles decorated with cathepsin-cleavable arginine-rich peptides functionalized with the near-infrared fluorochrome Cy5.5 (the peptides facilitate the uptake of the nanoparticles by early autophagosomes, and are then cleaved by cathepsins in lysosomes). In the heart tissue of live mice, the nanoparticles enabled quantitative measurements of changes in autophagic flux, upregulated genetically, by ischaemia-reperfusion injury or via starvation, or inhibited via the administration of a chemotherapeutic or the antibiotic bafilomycin. In mice receiving doxorubicin, pre-starvation improved cardiac function and overall survival, suggesting that bursts of increased autophagic flux may have cardioprotective effects during chemotherapy. Autophagy-detecting nanoparticle probes may facilitate the further understanding of the roles of autophagy in disease.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

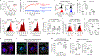

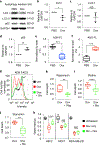
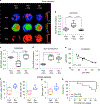

Comment in
-
Non-invasive monitoring of autophagy.Nat Biomed Eng. 2022 Sep;6(9):1015-1016. doi: 10.1038/s41551-022-00943-w. Nat Biomed Eng. 2022. PMID: 36127452 No abstract available.
Similar articles
-
Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes.Bioconjug Chem. 2002 May-Jun;13(3):554-60. doi: 10.1021/bc015555d. Bioconjug Chem. 2002. PMID: 12009946
-
Downregulation of LAPTM4B Contributes to the Impairment of the Autophagic Flux via Unopposed Activation of mTORC1 Signaling During Myocardial Ischemia/Reperfusion Injury.Circ Res. 2020 Sep 11;127(7):e148-e165. doi: 10.1161/CIRCRESAHA.119.316388. Epub 2020 Jul 22. Circ Res. 2020. PMID: 32693673
-
Near-Infrared Fluorescent and Magnetic Resonance Dual-Imaging Coacervate Nanoprobes for Trypsin Mapping and Targeted Payload Delivery of Malignant Tumors.ACS Appl Mater Interfaces. 2020 Apr 15;12(15):17302-17313. doi: 10.1021/acsami.0c03433. Epub 2020 Apr 2. ACS Appl Mater Interfaces. 2020. PMID: 32212678
-
In vivo Near-infrared Fluorescence Tumor Imaging Using DiR-loaded Nanocarriers.Curr Drug Deliv. 2016;13(1):40-8. doi: 10.2174/1567201812666150703114908. Curr Drug Deliv. 2016. PMID: 26138681 Review.
-
In vivo near-infrared fluorescence imaging of cancer with nanoparticle-based probes.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 Jul-Aug;2(4):349-66. doi: 10.1002/wnan.85. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010. PMID: 20564463 Review.
Cited by
-
Application of Nanomaterials and Related Drug Delivery Systems in Autophagy.Molecules. 2024 Jul 26;29(15):3513. doi: 10.3390/molecules29153513. Molecules. 2024. PMID: 39124918 Free PMC article. Review.
-
Advanced Nanoprobe Strategies for Imaging Macrophage Polarization in Cancer Immunology.Research (Wash D C). 2025 Feb 21;8:0622. doi: 10.34133/research.0622. eCollection 2025. Research (Wash D C). 2025. PMID: 39990770 Free PMC article. Review.
-
Ischemia-induced cardiac dysfunction is exacerbated in adiponectin-knockout mice due to impaired autophagy flux.Clin Transl Sci. 2024 Mar;17(3):e13758. doi: 10.1111/cts.13758. Clin Transl Sci. 2024. PMID: 38515365 Free PMC article.
-
Biological Properties of Arginine-rich Peptides and their Application in Cargo Delivery to Cancer.Curr Drug Deliv. 2025;22(4):387-400. doi: 10.2174/1567201820666230417083350. Curr Drug Deliv. 2025. PMID: 37073158 Review.
-
Let's talk about flux: the rising potential of autophagy rate measurements in disease.Autophagy. 2024 Nov;20(11):2574-2580. doi: 10.1080/15548627.2024.2371708. Epub 2024 Jul 10. Autophagy. 2024. PMID: 38984617 Free PMC article.
References
-
- Levine B & Klionsky DJ Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 (2004). - PubMed
-
- Levine B Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162 (2005). - PubMed
-
- Choi AM, Ryter SW & Levine B Autophagy in human health and disease. N. Engl. J. Med 368, 651–662 (2013). - PubMed
-
- Nishino I et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406, 906–910 (2000). - PubMed
-
- Mizushima N & Levine B Autophagy in human diseases. N. Engl. J. Med 383, 1564–1576 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

