Evidence based recommendations for an optimal prenatal supplement for women in the US: vitamins and related nutrients
- PMID: 35818085
- PMCID: PMC9275129
- DOI: 10.1186/s40748-022-00139-9
Evidence based recommendations for an optimal prenatal supplement for women in the US: vitamins and related nutrients
Abstract
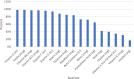
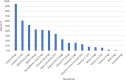
The blood levels of most vitamins decrease during pregnancy if un-supplemented, including vitamins A, C, D, K, B1, B3, B5, B6, folate, biotin, and B12. Sub-optimal intake of vitamins from preconception through pregnancy increases the risk of many pregnancy complications and infant health problems. In the U.S., dietary intake of vitamins is often below recommended intakes, especially for vitamin D, choline and DHA. Many studies suggest that insufficient vitamin intake is associated with a wide range of pregnancy complications (anemia, Cesarean section, depression, gestational diabetes, hypertension, infertility, preeclampsia, and premature rupture of membranes) and infant health problems (asthma/wheeze, autism, low birth weight, congenital heart defects, intellectual development, intrauterine growth restriction, miscarriage, neural tube defects, orofacial defects, and preterm birth). The primary goal of this paper is to review the research literature and propose evidence-based recommendations for the optimal level of prenatal supplementation for each vitamin for most women in the United States. A secondary goal was to compare these new recommendations with the levels of vitamins in over 180 commercial prenatal supplements. The analysis found that prenatal supplements vary widely in content, often contained only a subset of essential vitamins, and the levels were often below our recommendations. This suggests that increasing prenatal vitamin supplementation to the levels recommended here may reduce the incidence of many pregnancy complications and infant health problems which currently occur.
Keywords: Folate; Pregnancy; Prenatal Supplements; Vitamin B12; Vitamin D; Vitamins.
© 2022. The Author(s).
Conflict of interest statement
JBA is the Chair of the Scientific Advisory Board of the Neurological Health Foundation (NHF), Chair of the NHF Task Force on Pregnancy-Safe Products, co-leader of the Scientific Advisory Board of the Autism Research Institute, member of the Scientific Advisory Board for an autism study of microbiota transplant for Finch Therapeutics, President of the Autism Nutrition Research Center, President of Autism Diagnostics, President of Autism Therapeutics, and President of the Autism Society of Greater Phoenix. He has received grant funding from the Department of Defense, Finch Therapeutics, Autism Research Institute, Autism Nutrition Research Center, and BHARE Foundation. He is a paid consultant for Finch Therapeutics and Healthy Nest, and in the latter case he led the development of their prenatal supplement. He has patents/pending patents licensed to Finch Therapeutics and Autism Diagnostics. All authors have a pending patent on prenatal supplements.
Figures
Similar articles
-
Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals.Nutrients. 2021 May 28;13(6):1849. doi: 10.3390/nu13061849. Nutrients. 2021. PMID: 34071548 Free PMC article. Review.
-
Influence of mineral and vitamin supplements on pregnancy outcome.Eur J Obstet Gynecol Reprod Biol. 2012 Oct;164(2):127-32. doi: 10.1016/j.ejogrb.2012.06.020. Epub 2012 Jul 6. Eur J Obstet Gynecol Reprod Biol. 2012. PMID: 22771225 Review.
-
Pregnant Canadian Women Achieve Recommended Intakes of One-Carbon Nutrients through Prenatal Supplementation but the Supplement Composition, Including Choline, Requires Reconsideration.J Nutr. 2015 Aug;145(8):1824-34. doi: 10.3945/jn.115.211300. Epub 2015 Jun 10. J Nutr. 2015. PMID: 26063067
-
Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies.J Obstet Gynaecol Can. 2015 Jun;37(6):534-52. doi: 10.1016/s1701-2163(15)30230-9. J Obstet Gynaecol Can. 2015. PMID: 26334606 English, French.
-
Diet in Early Pregnancy: Focus on Folate, Vitamin B12, Vitamin D, and Choline.Can J Diet Pract Res. 2020 Jun 1;81(2):58-65. doi: 10.3148/cjdpr-2019-025. Epub 2019 Sep 12. Can J Diet Pract Res. 2020. PMID: 31512510
Cited by
-
A critical evaluation of prenatal supplements: Are they meeting the mark?J Family Med Prim Care. 2023 Dec;12(12):3048-3054. doi: 10.4103/jfmpc.jfmpc_1038_23. Epub 2023 Dec 21. J Family Med Prim Care. 2023. PMID: 38361856 Free PMC article. Review.
-
The effects of early childhood probiotic intake on the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children: a four-way decomposition analysis.Front Nutr. 2025 May 21;12:1614820. doi: 10.3389/fnut.2025.1614820. eCollection 2025. Front Nutr. 2025. PMID: 40469665 Free PMC article.
-
Plasma trimethylamine N-oxide metabolites in the second trimester predict the risk of hypertensive disorders of pregnancy: a nested case-control study.Hypertens Res. 2024 Mar;47(3):778-789. doi: 10.1038/s41440-023-01563-w. Epub 2024 Jan 4. Hypertens Res. 2024. PMID: 38177285
-
The Association Between Periconceptual Maternal Dietary Patterns and Miscarriage Risk in Women With Recurrent Miscarriages: A Multicentre Cohort Study.BJOG. 2025 Mar;132(4):504-517. doi: 10.1111/1471-0528.18022. Epub 2024 Nov 26. BJOG. 2025. PMID: 39588707 Free PMC article.
-
Association of vitamin A with gestational diabetes and thyroid disorders in pregnancy and their influence on maternal, fetal, and neonatal outcomes.Ther Adv Reprod Health. 2024 Aug 31;18:26334941241271542. doi: 10.1177/26334941241271542. eCollection 2024 Jan-Dec. Ther Adv Reprod Health. 2024. PMID: 39220467 Free PMC article. Review.
References
-
- Prevention CfDCa . Reproductive health data and statistics. 2020.
-
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. The official organ of the International Federation of Gynaecology and Obstetrics. Int J Gynaecol Obstet. 2022;78(2):179–90. 10.1016/s0020-7292(02)00197-2. - PubMed
Publication types
LinkOut - more resources
Full Text Sources