A Randomized Trial of Telephone-Based Smoking Cessation Treatment in the Lung Cancer Screening Setting
- PMID: 35818122
- PMCID: PMC9552302
- DOI: 10.1093/jnci/djac127
A Randomized Trial of Telephone-Based Smoking Cessation Treatment in the Lung Cancer Screening Setting
Abstract
Background: Lung cancer mortality is reduced via low-dose computed tomography screening and treatment of early-stage disease. Evidence-based smoking cessation treatment in the lung screening setting can further reduce mortality. We report the results of a cessation trial from the National Cancer Institute's Smoking Cessation at Lung Examination collaboration.
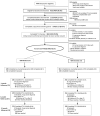
Methods: Eligible patients (n = 818) aged 50-80 years were randomly assigned (May 2017-January 2021) to the intensive vs minimal arms (8 vs 3 phone sessions plus 8 vs 2 weeks of nicotine patches, respectively). Bio-verified (primary) and self-reported 7-day abstinence rates were assessed at 3, 6, and 12 months post random assignment. Logistic regression analyses evaluated the effects of study arm. All statistical tests were 2-sided.
Results: Participants reported 48.0 (SD = 17.2) pack-years, and 51.6% were not ready to quit in less than 30 days. Self-reported 3-month quit rates were statistically significantly higher in the intensive vs minimal arm (14.3% vs 7.9%; odds ratio [OR] = 2.00, 95% confidence interval [CI] = 1.26 to 3.18). Bio-verified abstinence was lower but with similar relative differences between arms (9.1% vs 3.9%; OR = 2.70, 95% CI = 1.44 to 5.08). Compared with the minimal arm, the intensive arm was more effective among those with greater nicotine dependence (OR = 3.47, 95% CI = 1.55 to 7.76), normal screening results (OR = 2.58, 95% CI = 1.32 to 5.03), high engagement in counseling (OR = 3.03, 95% CI = 1.50 to 6.14), and patch use (OR = 2.81, 95% CI = 1.39 to 5.68). Abstinence rates did not differ statistically significantly between arms at 6 months (OR = 1.2, 95% CI = 0.68 to 2.11) or 12 months (OR = 1.4, 95% CI = 0.82 to 2.42).
Conclusions: Delivering intensive telephone counseling and nicotine replacement with lung screening is an effective strategy to increase short-term smoking cessation. Methods to maintain short-term effects are needed. Even with modest quit rates, integrating cessation treatment into lung screening programs may have a large impact on tobacco-related mortality.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- van der Aalst CM, de Koning HJ, van den Bergh KAM, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer Amst Neth. 2012;76(2):204–210. doi:10.1016/j.lungcan.2011.10.006 - DOI - PubMed