SARS-CoV-2 Brain Regional Detection, Histopathology, Gene Expression, and Immunomodulatory Changes in Decedents with COVID-19
- PMID: 35818336
- PMCID: PMC9278252
- DOI: 10.1093/jnen/nlac056
SARS-CoV-2 Brain Regional Detection, Histopathology, Gene Expression, and Immunomodulatory Changes in Decedents with COVID-19
Abstract
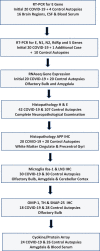
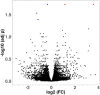
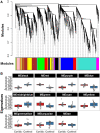
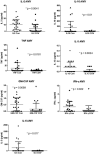
Brains of 42 COVID-19 decedents and 107 non-COVID-19 controls were studied. RT-PCR screening of 16 regions from 20 COVID-19 autopsies found SARS-CoV-2 E gene viral sequences in 7 regions (2.5% of 320 samples), concentrated in 4/20 subjects (20%). Additional screening of olfactory bulb (OB), amygdala (AMY) and entorhinal area for E, N1, N2, RNA-dependent RNA polymerase, and S gene sequences detected one or more of these in OB in 8/21 subjects (38%). It is uncertain whether these RNA sequences represent viable virus. Significant histopathology was limited to 2/42 cases (4.8%), one with a large acute cerebral infarct and one with hemorrhagic encephalitis. Case-control RNAseq in OB and AMY found more than 5000 and 700 differentially expressed genes, respectively, unrelated to RT-PCR results; these involved immune response, neuronal constituents, and olfactory/taste receptor genes. Olfactory marker protein-1 reduction indicated COVID-19-related loss of OB olfactory mucosa afferents. Iba-1-immunoreactive microglia had reduced area fractions in cerebellar cortex and AMY, and cytokine arrays showed generalized downregulation in AMY and upregulation in blood serum in COVID-19 cases. Although OB is a major brain portal for SARS-CoV-2, COVID-19 brain changes are more likely due to blood-borne immune mediators and trans-synaptic gene expression changes arising from OB deafferentation.
Keywords: Amygdala; Cytokine; Deafferentation; Encephalitis; Microglia; Olfactory bulb; SARS-Cov-2.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Association of Neuropathologists, Inc. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures













References
-
- Delamarre L, Gollion C, Grouteau G, et al. ; NeuroICU Research Group. COVID-19-associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatry 2020;91:1004–6 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

