Evaluation of potential anti-metastatic and antioxidative abilities of natural peptides derived from Tecoma stans (L.) Juss. ex Kunth in A549 cells
- PMID: 35818360
- PMCID: PMC9270879
- DOI: 10.7717/peerj.13693
Evaluation of potential anti-metastatic and antioxidative abilities of natural peptides derived from Tecoma stans (L.) Juss. ex Kunth in A549 cells
Abstract
Background: Tecoma stans (L.) Juss. ex Kunth is a well-known medicinal plant found in tropical and subtropical regions. It contains a broad range of bioactive compounds that exhibit many biological effects, including antidiabetic, antibacterial, and antioxidative activities. However, the effect of natural peptides from T. stans against cancer progression and free radical production is unknown. This study aims to evaluate the cytotoxic, anti-metastatic, and antioxidative activities of natural peptides from T. stans on A549 cells.
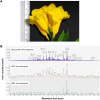
Methods: The natural peptides were extracted from the flower of T. stans using the pressurized hot water extraction (PHWE) method, followed by size exclusion chromatography and solid-phase extraction-C18. The cytotoxic and anti-metastatic effects of natural peptides were evaluated using MTT and transwell chamber assays, respectively. The free radical scavenging activity of natural peptides was determined using ABTS, DPPH, and FRAP assays. The cells were pretreated with the IC50 dosage of natural peptides and stimulated with LPS before analyzing intracellular reactive oxygen species (ROS) and proteomics.
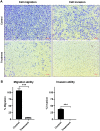
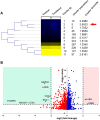
Results: Natural peptides induced cell toxicity at a concentration of less than 1 ng/ml and markedly reduced cell motility of A549 cells. The cells had a migration rate of less than 10% and lost their invasion ability in the treatment condition. In addition, natural peptides showed free radical scavenging activity similar to standard antioxidants and significantly decreased intracellular ROS in the LPS-induced cells. Proteomic analysis revealed 1,604 differentially expressed proteins. The self-organizing tree algorithm (SOTA) clustered the protein abundances into eleven groups. The volcano plot revealed that the cancer-promoting proteins (NCBP2, AMD, MER34, ENC1, and COA4) were down-regulated, while the secretory glycoprotein (A1BG) and ROS-reducing protein (ASB6) were up-regulated in the treatment group.
Conclusion: The anti-proliferative and anti-metastatic activities of natural peptides may be attributed to the suppression of several cancer-promoting proteins. In contrast, their antioxidative activity may result from the up-regulation of ROS-reducing protein. This finding suggests that natural peptides from T. stans are viable for being the new potential anti-cancer and antioxidative agents.
Keywords: Anti-metastasis; Antioxidant; Medicinal plant; Natural peptide; Proteomics.
© 2022 Krobthong et al.
Conflict of interest statement
Wattanapong Sittisaree is employed by Merck Life Science Thailand, Merck Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A review on phytochemistry and pharmacological uses of Tecoma stans (L.) Juss. ex Kunth.J Ethnopharmacol. 2021 Jan 30;265:113270. doi: 10.1016/j.jep.2020.113270. Epub 2020 Aug 18. J Ethnopharmacol. 2021. PMID: 32822823 Review.
-
Protective effect of Tecoma stans (L.) Juss.ex Kunth in CFA-induced arthritic rats.J Ethnopharmacol. 2025 Jan 30;337(Pt 3):118944. doi: 10.1016/j.jep.2024.118944. Epub 2024 Oct 16. J Ethnopharmacol. 2025. PMID: 39423943
-
Chemical composition, antioxidant and cytotoxic activities of extracts from the pericarp of Tecoma stans (L.) Juss. Ex Kunth (Bignoniaceae).Nat Prod Res. 2023 Jun;37(12):2070-2075. doi: 10.1080/14786419.2022.2116702. Epub 2022 Aug 26. Nat Prod Res. 2023. PMID: 36028333
-
Anti-Zika virus activity and chemical characterization by ultra-high performance liquid chromatography (UPLC-DAD-UV-MS) of ethanol extracts in Tecoma species.BMC Complement Med Ther. 2020 Aug 7;20(1):246. doi: 10.1186/s12906-020-03040-0. BMC Complement Med Ther. 2020. PMID: 32767975 Free PMC article.
-
A comprehensive review on ecology, life cycle and use of Tecoma stans (bignoneaceae).Bot Stud. 2024 Feb 13;65(1):6. doi: 10.1186/s40529-024-00412-4. Bot Stud. 2024. PMID: 38347314 Free PMC article. Review.
Cited by
-
Pretreatment with aqueous Moringa oleifera Lam. leaf extract prevents cadmium-induced hepatotoxicity by improving cellular antioxidant machinery and reducing cadmium accumulation.Heliyon. 2024 Sep 7;10(18):e37424. doi: 10.1016/j.heliyon.2024.e37424. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309955 Free PMC article.
-
Protective Effects of an Octapeptide Identified from Riceberry™ (Oryza sativa) Protein Hydrolysate on Oxidative and Endoplasmic Reticulum (ER) Stress in L929 Cells.Foods. 2024 Aug 5;13(15):2467. doi: 10.3390/foods13152467. Foods. 2024. PMID: 39123656 Free PMC article.
-
GNL3 and PA2G4 as Prognostic Biomarkers in Prostate Cancer.Cancers (Basel). 2023 May 11;15(10):2723. doi: 10.3390/cancers15102723. Cancers (Basel). 2023. PMID: 37345060 Free PMC article. Review.
-
Tandem Mass Tag-10plex (TMT-10plex) phosphoproteomics dataset for comprehensive analysis of active compounds with tyrosine kinase inhibition activity.Data Brief. 2024 May 29;55:110570. doi: 10.1016/j.dib.2024.110570. eCollection 2024 Aug. Data Brief. 2024. PMID: 38952951 Free PMC article.
References
-
- Abramovj H, Grobin B, Poklar Ulrih N, Cigj B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin Ciocalteu. Journal of Chemistry. 2018;2018:4608405. doi: 10.1155/2018/4608405. - DOI
-
- Alonso-Castro AJ, Zapata-Bustos R, Romo-Yañez J, Camarillo-Ledesma P, Gómez-Sánchez M, Salazar-Olivo LA. The antidiabetic plants Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) and Teucrium cubense Jacq (Lamiaceae) induce the incorporation of glucose in insulin-sensitive and insulin-resistant murine and human adipocytes. Journal of Ethnopharmacology. 2010;127(1):1–6. doi: 10.1016/j.jep.2009.09.060. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

