Cataracts in setting of multisystem inflammation after COVID-19 vaccination
- PMID: 35818371
- PMCID: PMC9259510
- DOI: 10.1016/j.ajoc.2022.101654
Cataracts in setting of multisystem inflammation after COVID-19 vaccination
Abstract
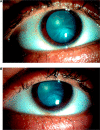
Purpose: To describe a unique case of bilateral cataract formation in the setting of multisystem inflammation after the 1st dose of the BNT162b2 mRNA COVID-19 vaccination.
Observations: A previously healthy 20-year-old male developed intumescent bilateral cataracts leading to visual decline from 20/20 to 20/300-20/400 in each eye, likely from systemic inflammation after vaccination.
Conclusion and importance: This is the first reported case of cataract formation following a COVID-19 vaccine. While ocular adverse effects associated with COVID-19 vaccination are rare, it is important to raise awareness of these entities amongst medical providers as the COVID-19 pandemic continues and vaccinations become widespread.
Keywords: COVID-19; Cataracts; Coronavirus; Multisystem inflammatory syndrome-vaccine; Post-vaccination inflammation.
© 2022 The Authors.
Conflict of interest statement
All authors have no financial disclosures.
Figures
References
-
- Shah A.P., Dzhaber D., Kenyon K.R., Riaz K.M., Ouano D.P., Koo E.H. 2021. Acute Corneal Transplant Rejection after COVID-19 Vaccination.www.corneajrnl.com - PubMed
Publication types
LinkOut - more resources
Full Text Sources