Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy
- PMID: 35818428
- PMCID: PMC9270644
- DOI: 10.1016/j.jgr.2021.08.003
Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy
Abstract
Background: In aged skin, reactive oxygen species (ROS) induces degradation of the extracellular matrix (ECM), leading to visible aging signs. Collagens in the ECM are cleaved by matrix metalloproteinases (MMPs). Syringaresinol (SYR), isolated from Panax ginseng berry, has various physiological activities, including anti-inflammatory action. However, the anti-aging effects of SYR via antioxidant and autophagy regulation have not been elucidated.
Methods: The preventive effect of SYR on skin aging was investigated in human HaCaT keratinocytes in the presence of H2O2, and the keratinocyte cells were treated with SYR (0-200 μg/mL). mRNA and protein levels of MMP-2 and -9 were determined by real-time PCR and Western blotting, respectively. Radical scavenging activity was researched by 2,2 diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assays. LC3B level was assessed by Western blotting and confocal microscopy.
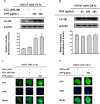
Results: SYR significantly reduced gene expression and protein levels of MMP-9 and -2 in both H2O2-treated and untreated HaCaT cells. SYR did not show cytotoxicity to HaCaT cells. SYR exhibited DPPH and ABTS radical scavenging activities with an EC50 value of 10.77 and 10.35 μg/mL, respectively. SYR elevated total levels of endogenous and exogenous LC3B in H2O2-stimulated HaCaT cells. 3-Methyladenine (3-MA), an autophagy inhibitor, counteracted the inhibitory effect of SYR on MMP-2 expression.
Conclusion: SYR showed antioxidant activity and up-regulated autophagy activity in H2O2-stimulated HaCaT cells, lowering the expression of MMP-2 and MMP-9 associated with skin aging. Our results suggest that SYR has potential value as a cosmetic additive for prevention of skin aging.
Keywords: 3-MA, 3-Methyladenine; ABTS, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid; Anti-aging; Antioxidant; Autophagy; CMA, chaperone-mediated autophagy; DMEM, Dulbecco's Modified Eagle's medium; DMSO, dimethyl sulfoxide; DPPH, 2,2 diphenyl-1-picrylhydrazyl; ECM, extracellular matrix; FBS, fetal bovine serum; FoxO3a, Forkhead box O3; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; Panax ginseng berry; ROS, reactive oxygen species; SYR, Syringaresinol; Syringaresinol; UV, ultraviolet.
© 2022 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

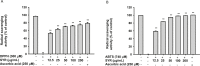


References
-
- Craven N.M., Watson R.E., Jones C.J., Shuttleworth C.A., Kielty C.M., Griffiths C.E. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br J Dermatol. 1997;137:344–350. - PubMed
-
- Talwar H.S., Griffiths C.E., Fisher G.J., Hamilton T.A., Voorhees J.J. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. - PubMed
-
- Kohl E., Steinbauer J., Landthaler M., Szeimies R.M. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25:873–884. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

