Cardiovascular Disease in Duchenne Muscular Dystrophy: Overview and Insight Into Novel Therapeutic Targets
- PMID: 35818510
- PMCID: PMC9270569
- DOI: 10.1016/j.jacbts.2021.11.004
Cardiovascular Disease in Duchenne Muscular Dystrophy: Overview and Insight Into Novel Therapeutic Targets
Abstract
Duchenne muscular dystrophy (DMD) is a devastating disease affecting approximately 1 in every 3,500 male births worldwide. Multiple mutations in the dystrophin gene have been implicated as underlying causes of DMD. However, there remains no cure for patients with DMD, and cardiomyopathy has become the most common cause of death in the affected population. Extensive research is under way investigating molecular mechanisms that highlight potential therapeutic targets for the development of pharmacotherapy for DMD cardiomyopathy. In this paper, the authors perform a literature review reporting on recent ongoing efforts to identify novel therapeutic strategies to reduce, prevent, or reverse progression of cardiac dysfunction in DMD.
Keywords: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ApN, adiponectin; BB, beta-blocker; BDNF, brain-derived neurotrophic factor; CMR, cardiac magnetic resonance imaging; Cx, connexin; DMD, Duchenne muscular dystrophy; DPC, dystrophin-associated protein complex; Duchenne muscular dystrophy; FFA, free fatty acid; HF, heart failure; LNP, lipid nanoparticle; LV, left ventricular; LVEF, left ventricular ejection fraction; NIV, noninvasive ventilation; Nrf2, nuclear factor erythroid 2-related factor 2; PKA, protein kinase A; PTX3, pentraxin 3; Px, pannexin; RNP, ribonucleoprotein complexes; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; RyR2, ryanodine receptor isoform 2; SR, sarcoplasmic reticulum; TRPV2, transient receptor potential cation channel, subfamily V, member 2; TrkB, tyrosine kinase B; arrhythmias; cardiomyopathy; inflammatory modulators; miR, microRNA; myocardial fibrosis; sgRNA, single guide RNA.
© 2022 The Authors.
Conflict of interest statement
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grants R35HL155651 (Dr Salloum) and K08HL155852 (Dr Raucci). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
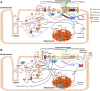
References
-
- Eiholzer U., Boltshauser E., Frey D., Molinari L., Zachmann M. Short stature: a common feature in Duchenne muscular dystrophy. Eur J Pediatr. 1988;147:602–605. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

