Major Adverse Cardiovascular Events in Patients With Renal Cell Carcinoma Treated With Targeted Therapies
- PMID: 35818552
- PMCID: PMC9270629
- DOI: 10.1016/j.jaccao.2022.05.002
Major Adverse Cardiovascular Events in Patients With Renal Cell Carcinoma Treated With Targeted Therapies
Abstract
Background: The risk for major adverse cardiovascular events (MACE) with targeted therapies for patients with advanced renal cell carcinoma (RCC) in real-world practice remains unclear.
Objectives: The aim of this study was to compare the risk for MACE associated with targeted cancer therapies with that associated with cytokine treatment in patients with advanced RCC.
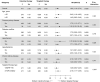
Methods: Using Taiwan's National Health Insurance Research Database, a retrospective nationwide cohort study was conducted involving patients with advanced RCC who had received targeted therapy (sunitinib, sorafenib, pazopanib, everolimus, or temsirolimus) or cytokine therapy (interleukin-2 or interferon gamma) from 2007 to 2018. Cox proportional hazards models were used to estimate the risk for MACE (a composite of myocardial infarction, ischemic stroke, heart failure, and cardiovascular death) in the cohort using the propensity score method of stabilized inverse probability of treatment weighting.
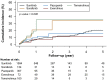
Results: In this cohort of 2,785 patients with advanced RCC, 2,257 (81%) and 528 (19%) had received targeted and cytokine therapy, respectively. After stabilized inverse probability of treatment weighting, the incidence rates of MACE were 6.65 and 3.36 per 100 person-years in the targeted and cytokine therapy groups, respectively (HR: 1.80; 95% CI: 1.19-2.74). Baseline history of heart failure (HR: 3.88; 95% CI: 2.25-6.71), atrial fibrillation (HR: 3.60; 95% CI: 2.16-5.99), venous thromboembolism (HR: 2.50; 95% CI: 1.27-4.92), ischemic stroke (HR: 1.88; 95% CI: 1.14-3.11), and age ≥ 65 years (HR: 1.81; 95% CI: 1.27-2.58) were independent risk factors for targeted therapy-associated MACE.
Conclusions: Among patients with advanced RCC, the risk for MACE associated with targeted cancer therapy is higher than that associated with cytokine therapy.
Keywords: CV, cardiovascular; GBM, generalized boosted model; MACE, major adverse cardiovascular event(s); NHIRD, National Health Insurance Research Database; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; cardiovascular toxicity; mTOR, mechanistic target of rapamycin; renal cell carcinoma; sIPTW, stabilized inverse probability of treatment weighting; targeted cancer therapy.
© 2022 The Authors.
Conflict of interest statement
This study was supported by Chang Gung Memorial Hospital (CMRPG3K0032). The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Zhu X., Stergiopoulos K., Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. - PubMed
-
- Wu S., Chen J.J., Kudelka A., Lu J., Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. - PubMed
-
- Choueiri T.K., Schutz F.A., Je Y., Rosenberg J.E., Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. - PubMed
-
- Qi W.X., Shen Z., Tang L.N., Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92:71–82. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

