Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment
- PMID: 35818571
- PMCID: PMC9258412
- DOI: 10.1016/j.omtm.2022.07.003
Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment
Abstract
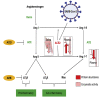
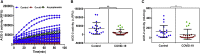
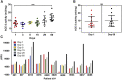
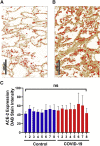
Although several therapeutics are used to treat coronavirus disease 2019 (COVID-19) patients, there is still no definitive metabolic marker to evaluate disease severity and recovery or a quantitative test to end quarantine. Because severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infects human cells via the angiotensin-converting-enzyme 2 (ACE2) receptor and COVID-19 is associated with renin-angiotensin system dysregulation, we evaluated soluble ACE2 (sACE2) activity in the plasma/saliva of 80 hospitalized COVID-19 patients and 27 non-COVID-19 volunteers, and levels of ACE2/Ang (1-7) in plasma or membrane (mACE2) in lung autopsy samples. sACE2 activity was markedly reduced (p < 0.0001) in COVID-19 plasma (n = 59) compared with controls (n = 27). Nadir sACE2 activity in early hospitalization was restored during disease recovery, irrespective of patient age, demographic variations, or comorbidity; in convalescent plasma-administered patients (n = 45), restoration was statistically higher than matched controls (n = 22, p = 0.0021). ACE2 activity was also substantially reduced in the saliva of COVID-19 patients compared with controls (p = 0.0065). There is a strong inverse correlation between sACE2 concentration and sACE2 activity and Ang (1-7) levels in participant plasmas. However, there were no difference in membrane ACE2 levels in lungs of autopsy tissues of COVID-19 (n = 800) versus other conditions (n = 300). These clinical observations suggest sACE2 activity as a potential biomarker and therapeutic target for COVID-19.
Keywords: COVID-19; SARS-CoV-2 diagnosis/treatment, soluble Angiotensin-Converting Enzyme 2, biomarker in plasma/saliva, Corona virus, COVID-19 disease, ACE2 activity/concentration, RAS pathway; angiotensin-converting-enzyme; biomarker; coronavirus disease; plasma marker; saliva marker.
© 2022 The Authors.
Conflict of interest statement
Authors declare no competing interests.
Figures


Similar articles
-
Potential detrimental role of soluble ACE2 in severe COVID-19 comorbid patients.Rev Med Virol. 2021 Sep;31(5):1-12. doi: 10.1002/rmv.2213. Epub 2021 Jan 10. Rev Med Virol. 2021. PMID: 33426683 Free PMC article. Review.
-
Sex difference in circulating soluble form of ACE2 protein in moderate and severe COVID-19 and healthy controls.Front Med (Lausanne). 2022 Dec 8;9:1058120. doi: 10.3389/fmed.2022.1058120. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36569121 Free PMC article.
-
Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers.J Med Virol. 2021 Oct;93(10):5908-5916. doi: 10.1002/jmv.27144. Epub 2021 Jul 6. J Med Virol. 2021. PMID: 34138483 Free PMC article.
-
Evaluation of angiotensin converting enzyme 2 (ACE2), angiotensin II (Ang II), miR-141-3p, and miR-421 levels in SARS-CoV-2 patients: a case-control study.BMC Infect Dis. 2024 Apr 22;24(1):429. doi: 10.1186/s12879-024-09310-3. BMC Infect Dis. 2024. PMID: 38649818 Free PMC article.
-
ACE2 Shedding and the Role in COVID-19.Front Cell Infect Microbiol. 2022 Jan 14;11:789180. doi: 10.3389/fcimb.2021.789180. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096642 Free PMC article. Review.
Cited by
-
Modulation of the pharmacokinetics of soluble ACE2 decoy receptors through glycosylation.Mol Ther Methods Clin Dev. 2024 Jul 19;32(3):101301. doi: 10.1016/j.omtm.2024.101301. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39185275 Free PMC article.
-
Levels of Angiotensin and Kinin Metabolite Peptides Related to COVID-19 Severity.ACS Pharmacol Transl Sci. 2023 Dec 13;7(1):186-194. doi: 10.1021/acsptsci.3c00227. eCollection 2024 Jan 12. ACS Pharmacol Transl Sci. 2023. PMID: 38230277 Free PMC article.
-
Renin-Angiotensin System and Sex Differences in COVID-19: A Critical Assessment.Circ Res. 2023 May 12;132(10):1320-1337. doi: 10.1161/CIRCRESAHA.123.321883. Epub 2023 May 11. Circ Res. 2023. PMID: 37167353 Free PMC article. Review.
-
Serum angiotensin-converting enzyme 2 as a potential biomarker for SARS-CoV-2 infection and vaccine efficacy.Front Immunol. 2022 Oct 12;13:1001951. doi: 10.3389/fimmu.2022.1001951. eCollection 2022. Front Immunol. 2022. PMID: 36311758 Free PMC article.
-
Immune system-related soluble mediators and COVID-19: basic mechanisms and clinical perspectives.Cell Commun Signal. 2022 Aug 29;20(1):131. doi: 10.1186/s12964-022-00948-7. Cell Commun Signal. 2022. PMID: 36038915 Free PMC article. Review.
References
-
- Chatterjee P., Gheblawi M., Wang K., Vu J., Kondaiah P., Oudit G.Y. Interaction between the apelinergic system and ACE2 in the cardiovascular system: therapeutic implications. Clin. Sci. (Lond). 2020;134:2319–2336. - PubMed
-
- Lores E., Wysocki J., Batlle D. ACE2, the kidney and the emergence of COVID-19 two decades after ACE2 discovery. Clin. Sci. (Lond.) 2020;134:2791–2805. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

