Clinical and Quality of Life Outcomes Following Temperature-Controlled Radiofrequency Neurolysis of the Posterior Nasal Nerve (RhinAer) for Treatment of Chronic Rhinitis
- PMID: 35818709
- PMCID: PMC9548948
- DOI: 10.1177/19458924221109987
Clinical and Quality of Life Outcomes Following Temperature-Controlled Radiofrequency Neurolysis of the Posterior Nasal Nerve (RhinAer) for Treatment of Chronic Rhinitis
Abstract
Background: Temperature-controlled radiofrequency (TCRF) neurolysis of the posterior nasal nerve (PNN; RhinAer) is a minimally invasive treatment option for patients with chronic rhinitis.
Objective: To determine clinical outcomes and quality of life (QoL) following TCRF neurolysis of the PNN.
Methods: A prospective single-arm study of 129 patients with chronic rhinitis at 16 medical centers in the United States and Germany.
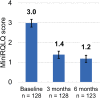
Results: The mean 24-h reflective total nasal symptom score (rTNSS) improved from 7.8 (95% CI, 7.5-8.1) at baseline to 3.6 (95% CI, 3.2-4.0) at 3 months and continued to improve to 2.9 (95% CI, 2.5-3.3) at 6 months (p < .001 comparing follow-up to baseline and p = .002 comparing 3 and 6 months). This represents 53.8% improvement over baseline at 3 months and 62.8% improvement at 6 months. Rhinorrhea, congestion, sneezing, and itching subscores and postnasal drip and cough scores were all significantly improved over baseline at both timepoints. At 3 months, 76.2% (95% CI, 68.1%-82.8%) of patients achieved a minimal clinically important difference of ≥30% improvement in rTNSS over baseline and the percentage was higher at 6 months (83.5% [95% CI, 75.8%-89.0%]). At 3 months, 80.3% (95% CI, 72.6%-86.3%) reported a minimal clinically important difference of ≥0.4-point improvement in the mini rhinoconjunctivitis quality of life questionnaire score, and the percentage was higher at 6 months; 87.7% (95% CI, 80.7%-92.4%). There were no serious adverse events with a relationship to the device/procedure reported through 6 months.
Conclusion: In this large, multicenter study, TCRF neurolysis of the PNN was safe and resulted in a significant reduction in rhinitis symptom burden at 3 months that was sustained/improved through 6 months. The majority of patients reported a clinically relevant improvement in QoL at 3 and 6 months postprocedure.
Keywords: MiniRQLQ; chronic rhinitis; neurolysis; posterior nasal nerve; quality of life; rTNSS; radiofrequency; temperature-controlled.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Detlef Brehmer has received research funding from Aerin Medical. Jivianne Lee and Daniel Charous are consultants to Aerin Medical. The other authors have no other funding, financial relationships, or conflicts of interest to disclose.
Figures






References
-
- Wise SK, Lin SY, Toskala E, et al.. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108-352. - PubMed
-
- Vandenplas O, Vinnikov D, Blanc PD, et al.. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6(4):1274-1286.e1279. - PubMed
-
- Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for Vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016;130(Suppl 4):S7-S28. - PubMed
-
- Kikawada T. Endoscopic posterior nasal neurectomy: an alternative to Vidian neurectomy. Oper Tech Otolayngol Head Neck Surg. 2007;18(4):297-301.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

