The Effects of Antioxidant Nutraceuticals on Cellular Sulfur Metabolism and Signaling
- PMID: 35819295
- PMCID: PMC9885552
- DOI: 10.1089/ars.2022.0077
The Effects of Antioxidant Nutraceuticals on Cellular Sulfur Metabolism and Signaling
Abstract
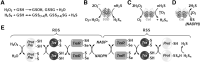
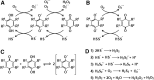
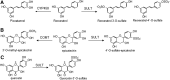
Significance: Nutraceuticals are ingested for health benefits, in addition to their general nutritional value. These dietary supplements have become increasingly popular since the late 20th century and they are a rapidly expanding global industry approaching a half-trillion U.S. dollars annually. Many nutraceuticals are promulgated as potent antioxidants. Recent Advances: Experimental support for the efficacy of nutraceuticals has lagged behind anecdotal exuberance. However, accumulating epidemiological evidence and recent, well-controlled clinical trials are beginning to support earlier animal and in vitro studies. Although still somewhat limited, encouraging results have been suggested in essentially all organ systems and against a wide range of pathophysiological conditions. Critical Issues: Health benefits of "antioxidant" nutraceuticals are largely attributed to their ability to scavenge oxidants. This has been criticized based on several factors, including limited bioavailability, short tissue retention time, and the preponderance of endogenous antioxidants. Recent attention has turned to nutraceutical activation of downstream antioxidant systems, especially the Keap1/Nrf2 (Kelch like ECH associated protein 1/nuclear factor erythroid 2-related factor 2) axis. The question now becomes, how do nutraceuticals activate this axis? Future Directions: Reactive sulfur species (RSS), including hydrogen sulfide (H2S) and its metabolites, are potent activators of the Keap1/Nrf2 axis and avid scavengers of reactive oxygen species. Evidence is beginning to accumulate that a variety of nutraceuticals increase cellular RSS by directly providing RSS in the diet, or through a number of catalytic mechanisms that increase endogenous RSS production. We propose that nutraceutical-specific targeting of RSS metabolism will lead to the design and development of even more efficacious antioxidant therapeutic strategies. Antioxid. Redox Signal. 38, 68-94.
Keywords: Nrf2; ROS; RSS; garlic; lipoic acid; oxidative stress; polyphenols; sulfur metabolism.
Conflict of interest statement
T.A.K., K.R.O., and P.J.D. have pending patents on the therapeutic use of polyphenols in regulating H2S metabolism. The other author has no competing financial interests that exist.
Figures





References
-
- Abrash HI, Shih D, Elias W, et al. A kinetic study of the air oxidation of pyrogallol and purpurogallin. Int J Chem Kinet 1989;21(6):465–466; doi: 10.1002/kin.550210609. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

