TMEM120A/TACAN inhibits mechanically activated PIEZO2 channels
- PMID: 35819364
- PMCID: PMC9280072
- DOI: 10.1085/jgp.202213164
TMEM120A/TACAN inhibits mechanically activated PIEZO2 channels
Abstract
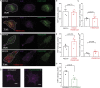
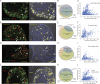
PIEZO2 channels mediate rapidly adapting mechanically activated currents in peripheral sensory neurons of the dorsal root ganglia (DRG), and they are indispensable for light touch and proprioception. Relatively little is known about what other proteins regulate PIEZO2 activity in a cellular context. TMEM120A (TACAN) was proposed to act as a high threshold mechanically activated ion channel in nociceptive DRG neurons. Here, we find that Tmem120a coexpression decreased the amplitudes of mechanically activated PIEZO2 currents and increased their threshold of activation. TMEM120A did not inhibit mechanically activated PIEZO1 and TREK1 channels and TMEM120A alone did not result in the appearance of mechanically activated currents above background. Tmem120a and Piezo2 expression in mouse DRG neurons overlapped, and siRNA-mediated knockdown of Tmem120a increased the amplitudes of rapidly adapting mechanically activated currents and decreased their thresholds to mechanical activation. Our data identify TMEM120A as a negative modulator of PIEZO2 channel activity, and do not support TMEM120A being a mechanically activated ion channel.
© 2022 Del Rosario et al.
Figures









References
-
- Assaraf, E., Blecher R., Heinemann-Yerushalmi L., Krief S., Carmel Vinestock R., Biton I.E., Brumfeld V., Rotkopf R., Avisar E., Agar G., and Zelzer E.. 2020. Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 11:3168. 10.1038/s41467-020-16971-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

