Dendritic spine morphology regulates calcium-dependent synaptic weight change
- PMID: 35819365
- PMCID: PMC9280073
- DOI: 10.1085/jgp.202112980
Dendritic spine morphology regulates calcium-dependent synaptic weight change
Abstract
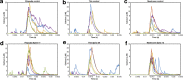
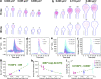
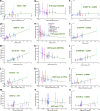
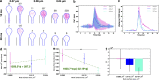
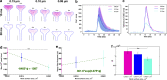
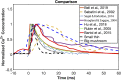
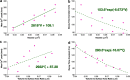
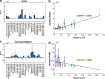
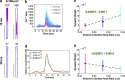
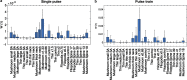
Dendritic spines act as biochemical computational units and must adapt their responses according to their activation history. Calcium influx acts as the first signaling step during postsynaptic activation and is a determinant of synaptic weight change. Dendritic spines also come in a variety of sizes and shapes. To probe the relationship between calcium dynamics and spine morphology, we used a stochastic reaction-diffusion model of calcium dynamics in idealized and realistic geometries. We show that despite the stochastic nature of the various calcium channels, receptors, and pumps, spine size and shape can modulate calcium dynamics and subsequently synaptic weight updates in a deterministic manner. Through a series of exhaustive simulations and analyses, we found that the calcium dynamics and synaptic weight change depend on the volume-to-surface area of the spine. The relationships between calcium dynamics and spine morphology identified in idealized geometries also hold in realistic geometries, suggesting that there are geometrically determined deterministic relationships that may modulate synaptic weight change.
© 2022 Bell et al.
Figures