Scalable, Chemoselective Nickel Electrocatalytic Sulfinylation of Aryl Halides with SO2
- PMID: 35819400
- PMCID: PMC9452475
- DOI: 10.1002/anie.202208080
Scalable, Chemoselective Nickel Electrocatalytic Sulfinylation of Aryl Halides with SO2
Abstract
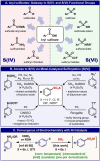
Simple access to aryl sulfinates from aryl iodides and bromides is reported using an inexpensive Ni-electrocatalytic protocol. The reaction exhibits a broad scope, uses stock solution of simple SO2 as sulfur source, and can be scaled up in batch and recycle flow settings. The limitations of this reaction are clearly shown and put into context by benchmarking with state-of-the-art Pd-based methods.
Keywords: Chemoselectivity; Electrochemistry; Fluoride; Nickel; Sulfur.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Chemoselective, Scalable Nickel-Electrocatalytic O-Arylation of Alcohols.Angew Chem Int Ed Engl. 2021 Sep 13;60(38):20700-20705. doi: 10.1002/anie.202107820. Epub 2021 Aug 15. Angew Chem Int Ed Engl. 2021. PMID: 34288303 Free PMC article.
-
Nickel-catalyzed electrochemical carboxylation of unactivated aryl and alkyl halides with CO2.Nat Commun. 2021 Dec 6;12(1):7086. doi: 10.1038/s41467-021-27437-8. Nat Commun. 2021. PMID: 34873172 Free PMC article.
-
Nickel-Electrocatalytic Decarboxylative Arylation to Access Quaternary Centers.Angew Chem Int Ed Engl. 2024 Feb 19;63(8):e202314617. doi: 10.1002/anie.202314617. Epub 2024 Jan 18. Angew Chem Int Ed Engl. 2024. PMID: 38181042
-
Efficient metal-free photochemical borylation of aryl halides under batch and continuous-flow conditions.Chem Sci. 2016 Jun 1;7(6):3676-3680. doi: 10.1039/c5sc04521e. Epub 2016 Feb 1. Chem Sci. 2016. PMID: 30008997 Free PMC article.
-
Chemoselective Reactions of Functionalized Sulfonyl Halides.Chem Rec. 2024 Feb;24(2):e202300256. doi: 10.1002/tcr.202300256. Epub 2023 Oct 12. Chem Rec. 2024. PMID: 37823680 Review.
Cited by
-
Nickel-Catalyzed Enantioselective Electrochemical Reductive Cross-Coupling of Aryl Aziridines with Alkenyl Bromides.J Am Chem Soc. 2023 Mar 22;145(11):6270-6279. doi: 10.1021/jacs.2c12869. Epub 2023 Mar 7. J Am Chem Soc. 2023. PMID: 36881734 Free PMC article.
-
One-Pot Synthesis of Sulfonamides from Unactivated Acids and Amines via Aromatic Decarboxylative Halosulfonylation.J Am Chem Soc. 2023 Oct 4;145(39):21189-21196. doi: 10.1021/jacs.3c08218. Epub 2023 Sep 20. J Am Chem Soc. 2023. PMID: 37729614 Free PMC article.
-
Interrogating the Mechanistic Features of Ni(I)-Mediated Aryl Iodide Oxidative Addition Using Electroanalytical and Statistical Modeling Techniques.J Am Chem Soc. 2023 Apr 4:10.1021/jacs.3c01726. doi: 10.1021/jacs.3c01726. Online ahead of print. J Am Chem Soc. 2023. PMID: 37014945 Free PMC article.
-
An organophotocatalytic redox-neutral strategy for late-stage drug functionalization with SO2 gas.Chem Sci. 2025 Feb 13;16(12):5064-5075. doi: 10.1039/d4sc08380f. eCollection 2025 Mar 19. Chem Sci. 2025. PMID: 39991557 Free PMC article.
-
Generation of perthiyl radicals for the synthesis of unsymmetric disulfides.Nat Commun. 2025 Jan 2;16(1):23. doi: 10.1038/s41467-024-55310-x. Nat Commun. 2025. PMID: 39747023 Free PMC article.
References
-
- Kleemann A., Engel J., Pharmaceutical Substances: Syntheses, Patents, Applications, Thieme, Stuttgart, 1999.
-
- McGrath N. A., Brichacek M., Njardarson J. T., J. Chem. Educ. 2010, 87, 1348–1349.
-
- Winum J.-Y., Scozzafava A., Montero J.-L., Supuran C. T., Med. Res. Rev. 2006, 26, 767–792. - PubMed
-
- Appleby A. P., Müller F., Carpy S., Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2001.
-
- Markwardt F., Drawert J., Walsmann P., Biochem. Pharmacol. 1974, 23, 2247–2256. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

