Content Validity of Patient-Reported Outcome Measures Developed for Assessing Health-Related Quality of Life in People with Type 2 Diabetes Mellitus: a Systematic Review
- PMID: 35819705
- PMCID: PMC9355936
- DOI: 10.1007/s11892-022-01482-z
Content Validity of Patient-Reported Outcome Measures Developed for Assessing Health-Related Quality of Life in People with Type 2 Diabetes Mellitus: a Systematic Review
Abstract
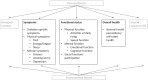
Purpose of review: We aimed to systematically evaluate the content validity of patient-reported outcome measures (PROMs) specifically developed to measure (aspects of) health-related quality of life (HRQOL) in people with type 2 diabetes. A systematic review was performed in PubMed and Embase of PROMs measuring perceived symptoms, physical function, mental function, social function/participation, and general health perceptions, and that were validated to at least some extent. Content validity (relevance, comprehensiveness, and comprehensibility) was evaluated using COSMIN methodology.
Recent findings: We identified 54 (different versions of) PROMs, containing 150 subscales. We found evidence for sufficient content validity for only 41/150 (27%) (subscales of) PROMs. The quality of evidence was generally very low. We found 66 out of 150 (44%) (subscales of) PROMs with evidence for either insufficient relevance, insufficient comprehensiveness, or insufficient comprehensibility. For measuring diabetes-specific symptoms, physical function, mental function, social function/participation, and general health perceptions, we identified one to 11 (subscales of) PROMs with sufficient content validity, although quality of the evidence was generally low. For measuring depressive symptoms, no PROM with sufficient content validity was identified. For each aspect of HRQL, we found at least one PROM with sufficient content validity, except for depressive symptoms. The quality of the evidence was mostly very low.
Keywords: Diabetes; Patient-reported outcomes; Quality of life; Questionnaire; Systematic review; Validity.
© 2022. The Author(s).
Conflict of interest statement
M de Wit was co-author on one of the included PROM development papers [71]. She was not involved in any of the ratings of this paper.
Figures
Similar articles
-
Patient-reported outcome measures for assessing health-related quality of life in people with type 2 diabetes: A systematic review.Rev Endocr Metab Disord. 2022 Oct;23(5):931-977. doi: 10.1007/s11154-022-09734-9. Epub 2022 Jul 2. Rev Endocr Metab Disord. 2022. PMID: 35779199 Free PMC article.
-
Patient-Reported Outcome Measures for Adults and Adolescents with Patellofemoral Pain: A Systematic Review of Content Validity and Feasibility Using the COSMIN Methodology.J Orthop Sports Phys Ther. 2023 Jan;53(1):23-39. doi: 10.2519/jospt.2022.11317. Epub 2022 Oct 17. J Orthop Sports Phys Ther. 2023. PMID: 36251651
-
A systematic review highlighting poor quality of evidence for content validity of quality of life instruments in female chronic pelvic pain.J Clin Epidemiol. 2022 Sep;149:1-11. doi: 10.1016/j.jclinepi.2022.04.016. Epub 2022 Apr 19. J Clin Epidemiol. 2022. PMID: 35452795
-
A systematic review highlights the need to investigate the content validity of patient-reported outcome measures for physical functioning in patients with low back pain.J Clin Epidemiol. 2018 Mar;95:73-93. doi: 10.1016/j.jclinepi.2017.11.005. Epub 2017 Nov 14. J Clin Epidemiol. 2018. PMID: 29154811
-
Physical activity for treatment of irritable bowel syndrome.Cochrane Database Syst Rev. 2022 Jun 29;6(6):CD011497. doi: 10.1002/14651858.CD011497.pub2. Cochrane Database Syst Rev. 2022. PMID: 35766861 Free PMC article.
Cited by
-
Measurement of compassion fatigue in animal health care professionals: a systematic review of available instruments and their content validity.Front Vet Sci. 2024 Jul 26;11:1425741. doi: 10.3389/fvets.2024.1425741. eCollection 2024. Front Vet Sci. 2024. PMID: 39132439 Free PMC article.
-
German Translation and Linguistic Validation of the LIMB‑Q: A Patient-reported Outcome Measure for Lower Extremity Trauma.Plast Reconstr Surg Glob Open. 2024 Jul 19;12(7):e6001. doi: 10.1097/GOX.0000000000006001. eCollection 2024 Jul. Plast Reconstr Surg Glob Open. 2024. PMID: 39036594 Free PMC article.
-
Patient-reported outcomes for people with diabetes: what and how to measure? A narrative review.Diabetologia. 2023 Aug;66(8):1357-1377. doi: 10.1007/s00125-023-05926-3. Epub 2023 May 24. Diabetologia. 2023. PMID: 37222772 Free PMC article. Review.
-
Systematic review on the measurement properties of diabetes-specific patient-reported outcome measures (PROMs) for measuring physical functioning in people with type 2 diabetes.BMJ Open Diabetes Res Care. 2022 Jun;10(3):e002729. doi: 10.1136/bmjdrc-2021-002729. BMJ Open Diabetes Res Care. 2022. PMID: 35675952 Free PMC article.
-
Patient-reported outcome measures for assessing health-related quality of life in people with type 2 diabetes: A systematic review.Rev Endocr Metab Disord. 2022 Oct;23(5):931-977. doi: 10.1007/s11154-022-09734-9. Epub 2022 Jul 2. Rev Endocr Metab Disord. 2022. PMID: 35779199 Free PMC article.
References
-
- Skovlund SE, Lichtenberg TH, Hessler D, Ejskjaer N. Can the routine use of patient-reported outcome measures improve the delivery of person-centered diabetes care? A review of recent developments and a case study. Curr DiabRep. 2019;19:84. - PubMed
-
- ICHOM Diabetes in Adults Working Group. Type 1 and type 2
-
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials