Economic Evaluation of Cost and Time Required for a Platform Trial vs Conventional Trials
- PMID: 35819785
- PMCID: PMC9277502
- DOI: 10.1001/jamanetworkopen.2022.21140
Economic Evaluation of Cost and Time Required for a Platform Trial vs Conventional Trials
Abstract
Importance: Platform trial design allows the introduction of new interventions after the trial is initiated and offers efficiencies to clinical research. However, limited guidance exists on the economic resources required to establish and maintain platform trials.
Objective: To compare cost (US dollars) and time requirements of conducting a platform trial vs a series of conventional (nonplatform) trials using a real-life example.
Design, setting, and participants: For this economic evaluation, an online survey was administered to a group of international experts (146 participants) with publication records of platform trials to elicit their opinions on cost and time to set up and conduct platform, multigroup, and 2-group trials. Using the reported entry dates of 10 interventions into Systemic Therapy in Advancing Metastatic Prostate Cancer: Evaluation of Drug Efficacy, the longest ongoing platform trial, 3 scenarios were designed involving a single platform trial (scenario 1), 1 multigroup followed by 5 2-group trials (scenario 2), and a series of 10 2-group trials (scenario 3). All scenarios started with 5 interventions, then 5 more interventions were either added to the platform or evaluated independently. Simulations with the survey results as inputs were used to compare the platform vs conventional trial designs. Data were analyzed from July to September 2021.
Exposure: Platform trial design.
Main outcomes and measures: Total trial setup and conduct cost and cumulative duration.
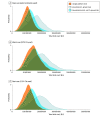
Results: Although setup time and cost requirements of a single trial were highest for the platform trial, cumulative requirements of setting up a series of multiple trials in scenarios 2 and 3 were larger. Compared with the platform trial, there was a median (IQR) increase of 216.7% (202.2%-242.5%) in cumulative setup costs for scenario 2 and 391.1% (365.3%-437.9%) for scenario 3. In terms of total cost, there was a median (IQR) increase of 17.4% (12.1%-22.5%) for scenario 2 and 57.5% (43.1%-69.9%) for scenario 3. There was a median (IQR) increase in cumulative trial duration of 171.1% (158.3%-184.3%) for scenario 2 and 311.9% (282.0%-349.1%) for scenario 3. Cost and time reductions in the platform trial were observed in both the initial and subsequently evaluated interventions.
Conclusions and relevance: Although setting up platform trials can take longer and be costly, the findings of this study suggest that having a single infrastructure can improve efficiencies with respect to costs and efforts.
Conflict of interest statement
Figures


Comment in
-
An Economic Perspective on Platform Trials-The Gift and the Curse.JAMA Netw Open. 2022 Jul 1;5(7):e2221149. doi: 10.1001/jamanetworkopen.2022.21149. JAMA Netw Open. 2022. PMID: 35819789 No abstract available.

