Cu-Catalyzed Chan-Evans-Lam Coupling Reactions of 2-Nitroimidazole with Aryl Boronic Acids: An Effort toward New Bioactive Agents against S. pneumoniae
- PMID: 35819995
- PMCID: PMC10184775
- DOI: 10.1002/cbdv.202200327
Cu-Catalyzed Chan-Evans-Lam Coupling Reactions of 2-Nitroimidazole with Aryl Boronic Acids: An Effort toward New Bioactive Agents against S. pneumoniae
Abstract
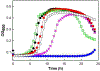
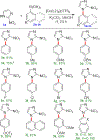
The coupling of phenylboronic acids with poorly-activated imidazoles is studied as a model system to explore the use of copper-catalyzed Chan-Evans-Lam (CEL) coupling for targeted C-N bond forming reactions. Optimized CEL reaction conditions are reported for four phenanthroline-based ligand systems, where the ligand 4,5-diazafluoren-9-one (dafo, L2) with 1 molar equivalent of potassium carbonate yielded the highest reactivity. The substrate 2-nitroimidazole (also known as azomycin) has documented antimicrobial activity against a range of microbes. Here N-arylation of 2-nitroimidazole with a range of aryl boronic acids has been successfully developed by copper(II)-catalyzed CEL reactions. Azomycin and a range of newly arylated azomycin derivatives were screened against S. pneumoniae, where 1-(4-(benzyloxy)phenyl)-2-nitro-1H-imidazole (3d) was demonstrated to have a minimal inhibition concentration value of 3.3 μg/mL.
Keywords: C−N bond forming reactions; S. pneumoniae; azomycin; copper(II); nitroimidazole.
© 2022 Wiley-VHCA AG, Zurich, Switzerland.
Figures




References
-
- Vitaku E, Smith DT, Njardarson JT, ‘Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals’, J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
- Yellol GS, Donaire A, Yellol JG, Vasylyeva V, Janiak C, Ruiz J, ‘On the Antitumor Properties of Novel Cyclometalated Benzimidazole Ru(II), Ir(III) and Rh(III) Complexes’, Chem. Commun 2013, 49, 11533–11535. - PubMed
-
- Lirag RC, Le HTM, Miljanić OŠ, ‘L-Shaped Benzimidazole Fluorophores: Synthesis, Characterization and Optical Response to Bases, Acids and Anions’, Chem. Commun 2013, 49, 4304–4306. - PubMed
-
- Jillella R, Raju S, Hsiao H-C, Hsu D-S, Chuang S-C, ‘Pd-Catalyzed Redox-Neutral C–N Coupling Reaction of Iminoquinones with Electron-Deficient Alkenes without External Oxidants: Access of Tertiary (E)-Enamines and Application to the Synthesis of Indoles and Quinolin-4-Ones’, Org. Lett 2020, 22, 6252–6256. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

