Cerebellar transcranial direct current stimulation disrupts neuroplasticity of intracortical motor circuits
- PMID: 35820111
- PMCID: PMC9275832
- DOI: 10.1371/journal.pone.0271311
Cerebellar transcranial direct current stimulation disrupts neuroplasticity of intracortical motor circuits
Abstract
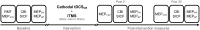
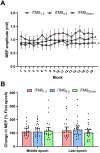
While previous research using transcranial magnetic stimulation (TMS) suggest that cerebellum (CB) influences the neuroplastic response of primary motor cortex (M1), the role of different indirect (I) wave inputs in M1 mediating this interaction remains unclear. The aim of this study was therefore to assess how CB influences neuroplasticity of early and late I-wave circuits. 22 young adults (22 ± 2.7 years) participated in 3 sessions in which I-wave periodicity repetitive transcranial magnetic stimulation (iTMS) was applied over M1 during concurrent application of cathodal transcranial direct current stimulation over CB (tDCSCB). In each session, iTMS either targeted early I-waves (1.5 ms interval; iTMS1.5), late I-waves (4.5 ms interval; iTMS4.5), or had no effect (variable interval; iTMSSham). Changes due to the intervention were examined with motor evoked potential (MEP) amplitude using TMS protocols measuring corticospinal excitability (MEP1mV) and the strength of CB-M1 connections (CBI). In addition, we indexed I-wave activity using short-interval intracortical facilitation (SICF) and low-intensity single-pulse TMS applied with posterior-anterior (MEPPA) and anterior-posterior (MEPAP) current directions. Following both active iTMS sessions, there was no change in MEP1mV, CBI or SICF (all P > 0.05), suggesting that tDCSCB broadly disrupted the excitatory response that is normally seen following iTMS. However, although MEPAP also failed to facilitate after the intervention (P > 0.05), MEPPA potentiated following both active iTMS sessions (both P < 0.05). This differential response between current directions could indicate a selective effect of CB on AP-sensitive circuits.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Update of
-
Characterising the influence of cerebellum on the neuroplastic modulation of intracortical motor circuits.PLoS One. 2020 Jul 10;15(7):e0236005. doi: 10.1371/journal.pone.0236005. eCollection 2020. PLoS One. 2020. Update in: PLoS One. 2022 Jul 12;17(7):e0271311. doi: 10.1371/journal.pone.0271311. PMID: 32649711 Free PMC article. Updated.
Similar articles
-
Modulation of dorsal premotor cortex differentially influences I-wave excitability in primary motor cortex of young and older adults.J Physiol. 2023 Jul;601(14):2959-2974. doi: 10.1113/JP284204. Epub 2023 May 29. J Physiol. 2023. PMID: 37194369 Free PMC article.
-
Effect of current direction and muscle activation on motor cortex neuroplasticity induced by repetitive paired-pulse transcranial magnetic stimulation.Eur J Neurosci. 2023 Sep;58(5):3270-3285. doi: 10.1111/ejn.16099. Epub 2023 Jul 27. Eur J Neurosci. 2023. PMID: 37501330 Free PMC article.
-
Modulation of I-Wave Generating Pathways With Repetitive Paired-Pulse Transcranial Magnetic Stimulation: A Transcranial Magnetic Stimulation-Electroencephalography Study.Neuromodulation. 2023 Jun;26(4):755-766. doi: 10.1016/j.neurom.2022.10.055. Epub 2022 Dec 1. Neuromodulation. 2023. PMID: 36463028
-
Interactions Between Cerebellum and the Intracortical Excitatory Circuits of Motor Cortex: a Mini-Review.Cerebellum. 2022 Feb;21(1):159-166. doi: 10.1007/s12311-021-01278-z. Epub 2021 May 12. Cerebellum. 2022. PMID: 33978934 Review.
-
Preferential Activation of Unique Motor Cortical Networks With Transcranial Magnetic Stimulation: A Review of the Physiological, Functional, and Clinical Evidence.Neuromodulation. 2021 Jul;24(5):813-828. doi: 10.1111/ner.13314. Epub 2020 Dec 9. Neuromodulation. 2021. PMID: 33295685 Review.
Cited by
-
Investigating the Effects of Repetitive Paired-Pulse Transcranial Magnetic Stimulation on Visuomotor Training Using TMS-EEG.Brain Topogr. 2024 Nov;37(6):1158-1170. doi: 10.1007/s10548-024-01071-1. Epub 2024 Jul 27. Brain Topogr. 2024. PMID: 39066878 Free PMC article.
-
Neuromodulation by repetitive paired-pulse TMS at late I-wave intervals in older adults.Exp Brain Res. 2025 May 6;243(6):140. doi: 10.1007/s00221-025-07060-5. Exp Brain Res. 2025. PMID: 40327107 Free PMC article.
-
Modulation of dorsal premotor cortex differentially influences I-wave excitability in primary motor cortex of young and older adults.J Physiol. 2023 Jul;601(14):2959-2974. doi: 10.1113/JP284204. Epub 2023 May 29. J Physiol. 2023. PMID: 37194369 Free PMC article.
-
Effect of current direction and muscle activation on motor cortex neuroplasticity induced by repetitive paired-pulse transcranial magnetic stimulation.Eur J Neurosci. 2023 Sep;58(5):3270-3285. doi: 10.1111/ejn.16099. Epub 2023 Jul 27. Eur J Neurosci. 2023. PMID: 37501330 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

