Moving Beyond Cyanoarene Thermally Activated Delayed Fluorescence Compounds as Photocatalysts: An Assessment of the Performance of a Pyrimidyl Sulfone Photocatalyst in Comparison to 4CzIPN
- PMID: 35820116
- PMCID: PMC10204087
- DOI: 10.1021/acs.joc.2c01137
Moving Beyond Cyanoarene Thermally Activated Delayed Fluorescence Compounds as Photocatalysts: An Assessment of the Performance of a Pyrimidyl Sulfone Photocatalyst in Comparison to 4CzIPN
Abstract
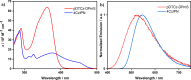
Carbazolyl dicyanobenzene (CDCB) derivates exhibiting thermally activated delayed fluorescence (TADF) have shown themselves to be excellent photocatalysts over recent years, particularly 4CzIPN, although investigation into organic TADF compounds as photocatalysts outside of the CDCB group has been limited. Herein, we report an alternative donor-acceptor TADF structure, 9,9'-(sulfonylbis(pyrimidine-5,2-diyl))bis(3,6-di-tert-butyl-9H-carbazole), pDTCz-DPmS, for use as a photocatalyst (PC). A comparison of the electrochemical and photophysical properties of pDTCz-DPmS with 4CzIPN in a range of solvents identifies the former as a better ground state reducing agent and photoreductant, while both exhibit similar oxidation capabilities in the ground and excited state. The increased conjugation of pDTCz-DPmS relative to 4CzIPN presents a more intense CT band in the UV-vis absorption spectrum, aiding in the light absorption of this molecule. Prompt and delayed emission lifetimes are observed for pDTCz-DPmS, confirming the TADF nature, both of which are sufficiently long-lived to participate in productive photochemistry. These combined properties make pDTCz-DPmS useful in photocatalysis reactions, covering a range of photoredox oxidative and reductive quenching reactions, as well as those involving a dual Ni(II) cocatalyst, alongside energy transfer processes. The higher triplet energy and increased photostability of pDTCz-DPmS compared with 4CzIPN were found to be advantages of this organic PC.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Noel T.; Zysman-Colman E. The Promise and Pitfalls of Photocatalysis for Organic Synthesis. Chem. Catal. 2022, 2, 468–476. 10.1016/j.checat.2021.12.015. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

