Building the phagocytic cup on an actin scaffold
- PMID: 35820329
- PMCID: PMC10078615
- DOI: 10.1016/j.ceb.2022.102112
Building the phagocytic cup on an actin scaffold
Abstract
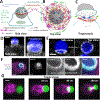
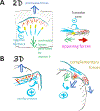
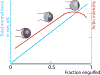
Cells ingest large particles, such as bacteria, viruses, or apoptotic cells, via the process of phagocytosis, which involves formation of an actin-rich structure known as the phagocytic cup. Phagocytic cup assembly and closure results from a concerted action of phagocytic receptors, regulators of actin polymerization, and myosin motors. Recent studies using advanced imaging approaches and biophysical techniques have revealed new information regarding phagocytic cup architecture, regulation of actin assembly, and the distribution, direction, and magnitude of the forces produced by the cytoskeletal elements that form the cup. These findings provide insights into the mechanisms leading to the assembly, expansion, and closure of phagocytic cups. The new data show that engulfment and internalization of phagocytic targets rely on several distinct yet complementary mechanisms that support the robust uptake of foreign objects and may be precisely tailored to the demands of specific phagocytic pathways.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Freeman SA, Grinstein S: Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 2014, 262:193–215. - PubMed
-
- Mattila PK, Lappalainen P: Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 2008, 9:446–454. - PubMed
-
- Mylvaganam S, Freeman SA, Grinstein S: The cytoskeleton in phagocytosis and macropinocytosis. Curr Biol 2021, 31:R619–R632. - PubMed
-
- Niedergang F, Grinstein S: How to build a phagosome: new concepts for an old process. Curr Opin Cell Biol 2018, 50:57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

