IFNγ-induced PD-L1 expression in ovarian cancer cells is regulated by JAK1, STAT1 and IRF1 signaling
- PMID: 35820543
- PMCID: PMC9357219
- DOI: 10.1016/j.cellsig.2022.110400
IFNγ-induced PD-L1 expression in ovarian cancer cells is regulated by JAK1, STAT1 and IRF1 signaling
Abstract
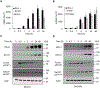
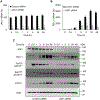
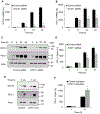
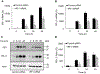
Expression of the immune checkpoint programmed death ligand-1 (PD-L1) is increased in ovarian cancer (OC) and correlates with poor prognosis. Interferon-γ (IFNγ) induces PD-L1 expression in OC cells, resulting in their increased proliferation and tumor growth, but the mechanisms that regulate the PD-L1 expression in OC remain unclear. Here, we show that the IFNγ-induced PD-L1 expression in OC cells is associated with increased levels of STAT1, Tyr-701 pSTAT1 and Ser-727 pSTAT1. Suppression of JAK1 and STAT1 significantly decreases the IFNγ-induced PD-L1 expression in OC cells, and STAT1 overexpression increases the IFNγ-induced PD-L1 expression. In addition, IFNγ induces expression of the transcription factor interferon regulatory factor 1 (IRF1) and IRF1 suppression attenuates the IFNγ-induced gene and protein levels of PD-L1. Chromatin immunoprecipitation results show that IFNγ induces PD-L1 promoter acetylation and recruitment of STAT1, Ser-727 pSTAT1 and IRF1 in OC cells. Together, these findings demonstrate that the IFNγ-induced PD-L1 expression in OC cells is regulated by JAK1, STAT1, and IRF1 signaling, and suggest that targeting the JAK1/ STAT1/IRF1 pathway may provide a leverage to regulate the PD-L1 levels in ovarian cancer.
Keywords: Interferon-γ; Ovarian cancer; PD-L1; STAT1; Transcriptional regulation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest
Figures
Similar articles
-
IFNγ-Induced Bcl3, PD-L1 and IL-8 Signaling in Ovarian Cancer: Mechanisms and Clinical Significance.Cancers (Basel). 2024 Jul 27;16(15):2676. doi: 10.3390/cancers16152676. Cancers (Basel). 2024. PMID: 39123403 Free PMC article. Review.
-
Immune checkpoint protein PD-L1 promotes transcription of angiogenic and oncogenic proteins IL-8, Bcl3, and STAT1 in ovarian cancer cells.J Biol Chem. 2025 Apr;301(4):108339. doi: 10.1016/j.jbc.2025.108339. Epub 2025 Feb 22. J Biol Chem. 2025. PMID: 39988077 Free PMC article.
-
Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression.Cell Rep. 2017 May 9;19(6):1189-1201. doi: 10.1016/j.celrep.2017.04.031. Cell Rep. 2017. PMID: 28494868 Free PMC article.
-
Differentiation status determines the effects of IFNγ on the expression of PD-L1 and immunomodulatory genes in melanoma.Cell Commun Signal. 2024 Dec 31;22(1):618. doi: 10.1186/s12964-024-01963-6. Cell Commun Signal. 2024. PMID: 39736644 Free PMC article.
-
Dual inhibition of STAT1 and STAT3 activation downregulates expression of PD-L1 in human breast cancer cells.Expert Opin Ther Targets. 2018 Jun;22(6):547-557. doi: 10.1080/14728222.2018.1471137. Epub 2018 May 2. Expert Opin Ther Targets. 2018. PMID: 29702007 Review.
Cited by
-
Identification of estrogen response-associated STRA6+ granulosa cells within high-grade serous ovarian carcinoma by single-cell sequencing.Heliyon. 2024 Mar 8;10(6):e27790. doi: 10.1016/j.heliyon.2024.e27790. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509903 Free PMC article.
-
Enhancing cancer care with improved checkpoint inhibitors: a focus on PD-1/PD-L1.EXCLI J. 2024 Oct 29;23:1303-1326. doi: 10.17179/excli2024-7783. eCollection 2024. EXCLI J. 2024. PMID: 39624113 Free PMC article. Review.
-
Functional gene signature offers a powerful tool for characterizing clinicopathological features and depicting tumor immune microenvironment of colorectal cancer.BMC Cancer. 2024 Sep 28;24(1):1199. doi: 10.1186/s12885-024-12996-y. BMC Cancer. 2024. PMID: 39342165 Free PMC article.
-
IFNγ-Induced Bcl3, PD-L1 and IL-8 Signaling in Ovarian Cancer: Mechanisms and Clinical Significance.Cancers (Basel). 2024 Jul 27;16(15):2676. doi: 10.3390/cancers16152676. Cancers (Basel). 2024. PMID: 39123403 Free PMC article. Review.
-
Malaria-derived hemozoin skews dendritic cell responses to bacterial infections by reducing interferon gene-transcription by SWI/SNF-NuRD.iScience. 2025 Jul 3;28(8):113046. doi: 10.1016/j.isci.2025.113046. eCollection 2025 Aug 15. iScience. 2025. PMID: 40717771 Free PMC article.
References
-
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al., Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 + T lymphocytes are prognostic factors of human ovarian cancer, Proc. Natl. Acad. Sd. U. S. A 104 (9) (2007) 3360–3365, 10.1073/pnas.0611533104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

