Dendritic involvement in inhibition and disinhibition of vulnerable dopaminergic neurons in healthy and pathological conditions
- PMID: 35820645
- PMCID: PMC9851599
- DOI: 10.1016/j.nbd.2022.105815
Dendritic involvement in inhibition and disinhibition of vulnerable dopaminergic neurons in healthy and pathological conditions
Abstract
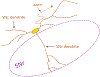
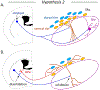
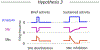
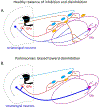
Dopaminergic neurons in the substantia nigra pars compacta (SNc) differentially degenerate in Parkinson's Disease, with the ventral region degenerating more severely than the dorsal region. Compared with the dorsal neurons, the ventral neurons in the SNc have distinct dendritic morphology, electrophysiological characteristics, and circuit connections with the basal ganglia. These characteristics shape information processing in the ventral SNc and structure the balance of inhibition and disinhibition in the striatonigral circuitry. In this paper, I review foundational studies and recent work comparing the circuitry of the ventral and dorsal SNc neurons and discuss how loss of the ventral neurons early in Parkinson's Disease could affect the overall balance of inhibition and disinhibition of dopamine signals.
Keywords: Basal ganglia; Circuit mapping; Dendrites; Dendritic morphology; Disinhibition; Dopamine; Inhibition; Nigrostriatal; Parkinson's disease; SNc; Striatonigral; Striatum; Substantia nigra.
Copyright © 2022 The Author. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures
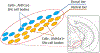

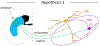
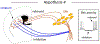
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

