Fever therapy in febrile adults: systematic review with meta-analyses and trial sequential analyses
- PMID: 35820685
- PMCID: PMC9274300
- DOI: 10.1136/bmj-2021-069620
Fever therapy in febrile adults: systematic review with meta-analyses and trial sequential analyses
Abstract
Objective: To investigate the effects of fever therapy compared with no fever therapy in a wide population of febrile adults.
Design: Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Data sources: CENTRAL, BIOSIS, CINAHL, MEDLINE, Embase, LILACS, Scopus, and Web of Science Core Collection, searched from their inception to 2 July 2021.
Eligibility criteria: Randomised clinical trials in adults diagnosed as having fever of any origin. Included experimental interventions were any fever therapy, and the control intervention had to be no fever therapy (with or without placebo/sham).
Data extraction and synthesis: Two authors independently selected studies, extracted data, and assessed the risk of bias. Primary outcomes were all cause mortality and serious adverse events. Secondary outcomes were quality of life and non-serious adverse events. Aggregate data were synthesised with meta-analyses, subgroup analyses, and trial sequential analyses, and the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Results: Forty two trials assessing 5140 participants were included. Twenty three trials assessed 11 different antipyretic drugs, 11 trials assessed physical cooling, and eight trials assessed a combination of antipyretic drugs and physical cooling. Of the participants, 3007 were critically ill, 1892 were non-critically ill, 3277 had infectious fever, and 1139 had non-infectious fever. All trials were assessed as being at high risk of bias. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of death (risk ratio 1.04, 95% confidence interval 0.90 to 1.19; I2=0%; P=0.62; 16 trials; high certainty evidence) and the risk of serious adverse events (risk ratio 1.02, 0.89 to 1.17; I2=0%; P=0.78; 16 trials; high certainty evidence) could be rejected. One trial assessing quality of life was included, showing no difference between fever therapy and control. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of non-serious adverse events could be neither confirmed nor rejected (risk ratio 0.92, 0.67 to 1.25; I2=66.5%; P=0.58; four trials; very low certainty evidence).
Conclusions: Fever therapy does not seem to affect the risk of death and serious adverse events.
Systematic review registration: PROSPERO CRD42019134006.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Swedish Research Council for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


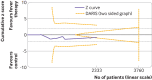

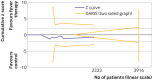
Comment in
-
In hospitalized adults with fever, fever therapy does not reduce mortality or serious adverse events.Ann Intern Med. 2022 Nov;175(11):JC127. doi: 10.7326/J22-0081. Epub 2022 Nov 1. Ann Intern Med. 2022. PMID: 36315945
Similar articles
-
Effect of level of sedation on outcomes in critically ill adult patients: a systematic review of clinical trials with meta-analysis and trial sequential analysis.EClinicalMedicine. 2024 Mar 28;71:102569. doi: 10.1016/j.eclinm.2024.102569. eCollection 2024 May. EClinicalMedicine. 2024. PMID: 38572080 Free PMC article.
-
Prophylactic drug management for febrile seizures in children.Cochrane Database Syst Rev. 2021 Jun 16;6(6):CD003031. doi: 10.1002/14651858.CD003031.pub4. Cochrane Database Syst Rev. 2021. PMID: 34131913 Free PMC article.
-
Beta-blockers for suspected or diagnosed acute myocardial infarction.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD012484. doi: 10.1002/14651858.CD012484.pub2. Cochrane Database Syst Rev. 2019. PMID: 31845756 Free PMC article.
-
Fever control interventions versus placebo, sham or no intervention in adults: a protocol for a systematic review with meta-analysis and Trial Sequential Analysis.BMJ Open. 2019 Nov 3;9(11):e032389. doi: 10.1136/bmjopen-2019-032389. BMJ Open. 2019. PMID: 31685514 Free PMC article.
-
N-acetylcysteine for non-paracetamol (acetaminophen)-related acute liver failure.Cochrane Database Syst Rev. 2020 Dec 9;12(12):CD012123. doi: 10.1002/14651858.CD012123.pub2. Cochrane Database Syst Rev. 2020. PMID: 33294991 Free PMC article.
Cited by
-
From fever to action: diagnosis, treatment, and prevention of acute undifferentiated febrile illnesses.Pathog Dis. 2024 Feb 7;82:ftae006. doi: 10.1093/femspd/ftae006. Pathog Dis. 2024. PMID: 38614961 Free PMC article. Review.
-
Comparative before-after study of fever prevention versus targeted temperature management following out-of-hospital cardiac arrest.Resusc Plus. 2023 Dec 21;17:100538. doi: 10.1016/j.resplu.2023.100538. eCollection 2024 Mar. Resusc Plus. 2023. PMID: 38205148 Free PMC article.
-
Temperature control in sepsis.Front Med (Lausanne). 2023 Oct 31;10:1292468. doi: 10.3389/fmed.2023.1292468. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38020082 Free PMC article. Review.
-
Clinical characteristics of perioperative central fever and its relationship with anesthesia.Medicine (Baltimore). 2023 Dec 15;102(50):e36523. doi: 10.1097/MD.0000000000036523. Medicine (Baltimore). 2023. PMID: 38115349 Free PMC article.
-
Clinical novel exploration of intractable fever in stroke rehabilitation: a single-center cross-sectional retrospective study.Sci Rep. 2025 Jan 22;15(1):2783. doi: 10.1038/s41598-025-87159-5. Sci Rep. 2025. PMID: 39843799 Free PMC article.
References
-
- Dai L, Zeng R. Fever. In: Wan X-H, Zeng R, eds. Handbook of Clinical Diagnostics. Springer Singapore, 2020: 3-7 10.1007/978-981-13-7677-1_1. - DOI
-
- Dinarello CA, Porat R. Pathophysiology and treatment of fever in adults. 2018 https://www.uptodate.com/contents/pathophysiology-and-treatment-of-fever....
-
- Balli S, Sharan S. Physiology, Fever. 2021. https://www.ncbi.nlm.nih.gov/books/NBK562334/. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
