Accurate liquid biopsy for the diagnosis of non-alcoholic steatohepatitis and liver fibrosis
- PMID: 35820779
- PMCID: PMC9872242
- DOI: 10.1136/gutjnl-2022-327498
Accurate liquid biopsy for the diagnosis of non-alcoholic steatohepatitis and liver fibrosis
Abstract
Objective: Clinical diagnosis and approval of new medications for non-alcoholic steatohepatitis (NASH) require invasive liver biopsies. The aim of our study was to identify non-invasive biomarkers of NASH and/or liver fibrosis.
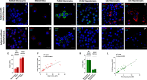
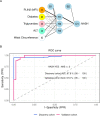
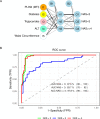
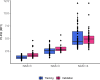
Design: This multicentre study includes 250 patients (discovery cohort, n=100 subjects (Bariatric Surgery Versus Non-alcoholic Steato-hepatitis - BRAVES trial); validation cohort, n=150 (Liquid Biopsy for NASH and Liver Fibrosis - LIBRA trial)) with histologically proven non-alcoholic fatty liver (NAFL) or NASH with or without fibrosis. Proteomics was performed in monocytes and hepatic stellate cells (HSCs) with iTRAQ-nano- Liquid Chromatography - Mass Spectrometry/Mass Spectrometry (LC-MS/MS), while flow cytometry measured perilipin-2 (PLIN2) and RAB14 in peripheral blood CD14+CD16- monocytes. Neural network classifiers were used to predict presence/absence of NASH and NASH stages. Logistic bootstrap-based regression was used to measure the accuracy of predicting liver fibrosis.
Results: The algorithm for NASH using PLIN2 mean florescence intensity (MFI) combined with waist circumference, triglyceride, alanine aminotransferase (ALT) and presence/absence of diabetes as covariates had an accuracy of 93% in the discovery cohort and of 92% in the validation cohort. Sensitivity and specificity were 95% and 90% in the discovery cohort and 88% and 100% in the validation cohort, respectively.The area under the receiver operating characteristic (AUROC) for NAS level prediction ranged from 83.7% (CI 75.6% to 91.8%) in the discovery cohort to 97.8% (CI 95.8% to 99.8%) in the validation cohort.The algorithm including RAB14 MFI, age, waist circumference, high-density lipoprotein cholesterol, plasma glucose and ALT levels as covariates to predict the presence of liver fibrosis yielded an AUROC of 95.9% (CI 87.9% to 100%) in the discovery cohort and 99.3% (CI 98.1% to 100%) in the validation cohort, respectively. Accuracy was 99.25%, sensitivity 100% and specificity 95.8% in the discovery cohort and 97.6%, 99% and 89.6% in the validation cohort. This novel biomarker was superior to currently used FIB4, non-alcoholic fatty liver disease fibrosis score and aspartate aminotransferase (AST)-to-platelet ratio and was comparable to ultrasound two-dimensional shear wave elastography.
Conclusions: The proposed novel liquid biopsy is accurate, sensitive and specific in diagnosing the presence and severity of NASH or liver fibrosis and is more reliable than currently used biomarkers.
Clinical trials: Discovery multicentre cohort: Bariatric Surgery versus Non-Alcoholic Steatohepatitis, BRAVES, ClinicalTrials.gov identifier: NCT03524365.Validation multicentre cohort: Liquid Biopsy for NASH and Fibrosis, LIBRA, ClinicalTrials.gov identifier: NCT04677101.
Keywords: HEPATIC FIBROSIS; NONALCOHOLIC STEATOHEPATITIS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GM reports consulting fees from Novo Nordisk,_Fractyl Inc and Recor Inc; she is also scientific current advisor and consultant of Metadeq Limited, and current advisor and consultant of Keyron Limited, GHP Scientific Limited, and Jemyll Limited. FR reports receiving research grants from Ethicon and Medtronic; consulting fees from Novo Nordisk, Ethicon and Medtronic; serving on scientific advisory boards for GI Dynamics; and is former director and current stock option holder of Metadeq Limited and former director and current advisor of Keyron Limited and GHP Scientific Limited. CWlR reports grants from the Irish Research Council, Science Foundation Ireland, Anabio and the Health Research Board; serves on advisory boards of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Sanofi Aventis, AstraZeneca, Janssen, Bristol-Myers Squibb, Glia and Boehringer Ingelheim. ClR is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here, and is the chief medical officer and director of the Medical Device Division of Keyron since January 2011; both of these are unremunerated positions. CWlR is also current director, shareholder and stock option holder of Metadeq Limited, current director of GHP Scientific Limited, was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. The product has only been tested in rodents and none of Keyron’s products are currently licensed. They do not have any contracts with other companies to put their products into clinical practice. No patients have been included in any of Keyron’s studies and they are not listed on the stock market. He continues to provide scientific advice to Keyron for no remuneration. All other authors declare no competing interests.
Figures
Comment in
-
Monocyte phenotypic liquid biopsy for NASH and liver fibrosis diagnosis: a new kid on the block.Gut. 2023 Dec 7;73(1):10-11. doi: 10.1136/gutjnl-2022-328189. Gut. 2023. PMID: 37328260 No abstract available.
References
-
- Pioglitazone vs vitamin E vs placebo for treatment of non-diabetic patients with nonalcoholic steatohepatitis (PIVENS). Available: https://clinicaltrials.gov/ct2/show/NCT00063622
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
