Dapagliflozin Prevents Kidney Glycogen Accumulation and Improves Renal Proximal Tubule Cell Functions in a Mouse Model of Glycogen Storage Disease Type 1b
- PMID: 35820785
- PMCID: PMC9528317
- DOI: 10.1681/ASN.2021070935
Dapagliflozin Prevents Kidney Glycogen Accumulation and Improves Renal Proximal Tubule Cell Functions in a Mouse Model of Glycogen Storage Disease Type 1b
Abstract
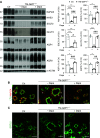
Background: Mutations in SLC37A4, which encodes the intracellular glucose transporter G6PT, cause the rare glycogen storage disease type 1b (GSD1b). A long-term consequence of GSD1b is kidney failure, which requires KRT. The main protein markers of proximal tubule function, including NaPi2A, NHE3, SGLT2, GLUT2, and AQP1, are downregulated as part of the disease phenotype.
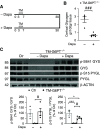
Methods: We utilized an inducible mouse model of GSD1b, TM-G6PT-/-, to show that glycogen accumulation plays a crucial role in altering proximal tubule morphology and function. To limit glucose entry into proximal tubule cells and thus to prevent glycogen accumulation, we administered an SGLT2-inhibitor, dapagliflozin, to TM-G6PT-/- mice.
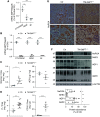
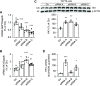
Results: In proximal tubule cells, G6PT suppression stimulates the upregulation and activity of hexokinase-I, which increases availability of the reabsorbed glucose for intracellular metabolism. Dapagliflozin prevented glycogen accumulation and improved kidney morphology by promoting a metabolic switch from glycogen synthesis toward lysis and by restoring expression levels of the main proximal tubule functional markers.
Conclusion: We provide proof of concept for the efficacy of dapagliflozin in preserving kidney function in GSD1b mice. Our findings could represent the basis for repurposing this drug to treat patients with GSD1b.
Keywords: NHE-3; Napi-2; SGLT-2 inhibitors; dapagliflozin; glycogen; glycogen storage disease 1b; hexokinase-1; proximal tubule.
Copyright © 2022 by the American Society of Nephrology.
Figures



References
-
- Melis D, Della Casa R, Parini R, Rigoldi M, Cacciapuoti C, Marcolongo P, et al. : Vitamin E supplementation improves neutropenia and reduces the frequency of infections in patients with glycogen storage disease type 1b. Eur J Pediatr 168: 1069–1074, 2009 - PubMed
-
- Visser G, Rake JP, Fernandes J, Labrune P, Leonard JV, Moses S, et al. : Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: Results of the European Study on Glycogen Storage Disease type I. J Pediatr 137: 187–191, 2000 - PubMed
-
- Visser G, Rake JP, Labrune P, Leonard JV, Moses S, Ullrich K, et al. : Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur J Pediatr 161[Suppl 1]: S83–S87, 2002 - PubMed
-
- Hiraiwa H, Pan CJ, Lin B, Moses SW, Chou JY: Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J Biol Chem 274: 5532–5536, 1999 - PubMed
-
- Chou JY, Matern D, Mansfield BC, Chen YT: Type I glycogen storage diseases: Disorders of the glucose-6-phosphatase complex. Curr Mol Med 2: 121–143, 2002 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

