New solid phase methodology for the synthesis of biscoumarin derivatives: experimental and in silico approaches
- PMID: 35820918
- PMCID: PMC9275028
- DOI: 10.1186/s13065-022-00844-8
New solid phase methodology for the synthesis of biscoumarin derivatives: experimental and in silico approaches
Abstract
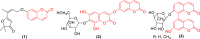
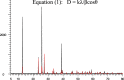
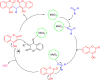
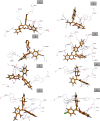
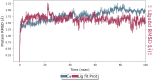
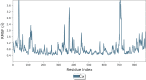
The simple and greener one-pot approach for the synthesis of biscoumarin derivatives using catalytic amounts of nano-MoO3 catalyst under mortar-pestle grinding was described. The use of non-toxic and mild catalyst, cost-effectiveness, ordinary grinding, and good to the excellent yield of the final product makes this procedure a more attractive pathway for the synthesis of biologically remarkable pharmacophores. Accordingly, biscoumarin derivatives were successfully extended in the developed protocols. Next, a computational investigation was performed to identify the potential biological targets of this set of compounds. In this case, first, a similarity search on different virtual libraries was performed to find an ideal biological target for these derivatives. Results showed that the synthesized derivatives can be α-glucosidase inhibitors. In another step, molecular docking studies were carried out against human lysosomal acid-alpha-glucosidase (PDB ID: 5NN8) to determine the detailed binding modes and critical interactions with the proposed target. In silico assessments showed the gold score value in the range of 17.56 to 29.49. Additionally, molecular dynamic simulations and the MM-GBSA method of the most active derivative against α-glucosidase were conducted to study the behavior of selected compounds in the biological system. Ligand 1 stabilized after around 30 ns and participated in various interactions with Trp481, Asp518, Asp616, His674, Phe649, and Leu677 residues.
Keywords: Biscoumarin; MM-GBSA; MoO3-nanoparticle; Molecular dynamics simulations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

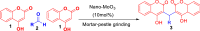

Similar articles
-
Synthesis and molecular docking studies of potent α-glucosidase inhibitors based on biscoumarin skeleton.Eur J Med Chem. 2014 Jun 23;81:245-52. doi: 10.1016/j.ejmech.2014.05.010. Epub 2014 May 4. Eur J Med Chem. 2014. PMID: 24844449
-
Biscoumarin-1,2,3-triazole hybrids as novel anti-diabetic agents: Design, synthesis, in vitro α-glucosidase inhibition, kinetic, and docking studies.Bioorg Chem. 2019 Nov;92:103206. doi: 10.1016/j.bioorg.2019.103206. Epub 2019 Aug 16. Bioorg Chem. 2019. PMID: 31445191
-
Investigation of the New Inhibitors by Sulfadiazine and Modified Derivatives of α-D-glucopyranoside for White Spot Syndrome Virus Disease of Shrimp by In Silico: Quantum Calculations, Molecular Docking, ADMET and Molecular Dynamics Study.Molecules. 2022 Jun 8;27(12):3694. doi: 10.3390/molecules27123694. Molecules. 2022. PMID: 35744817 Free PMC article.
-
Integrating Ligand and Target-Driven Based Virtual Screening Approaches With in vitro Human Cell Line Models and Time-Resolved Fluorescence Resonance Energy Transfer Assay to Identify Novel Hit Compounds Against BCL-2.Front Chem. 2020 Apr 9;8:167. doi: 10.3389/fchem.2020.00167. eCollection 2020. Front Chem. 2020. PMID: 32328476 Free PMC article.
-
Enzyme inhibitory activities an insight into the structure-Activity relationship of biscoumarin derivatives.Eur J Med Chem. 2017 Dec 1;141:386-403. doi: 10.1016/j.ejmech.2017.10.009. Epub 2017 Oct 6. Eur J Med Chem. 2017. PMID: 29032032 Review.
Cited by
-
Design, synthesis, and in silico studies of quinoline-based-benzo[d]imidazole bearing different acetamide derivatives as potent α-glucosidase inhibitors.Sci Rep. 2022 Aug 18;12(1):14019. doi: 10.1038/s41598-022-18455-7. Sci Rep. 2022. PMID: 35982225 Free PMC article.
-
Synthesis and bioactivities evaluation of quinazolin-4(3H)-one derivatives as α-glucosidase inhibitors.BMC Chem. 2022 Nov 15;16(1):97. doi: 10.1186/s13065-022-00885-z. BMC Chem. 2022. PMID: 36380337 Free PMC article.
-
Synthesis, in vitro inhibitor screening, structure-activity relationship, and molecular dynamic simulation studies of novel thioquinoline derivatives as potent α-glucosidase inhibitors.Sci Rep. 2023 May 15;13(1):7819. doi: 10.1038/s41598-023-35140-5. Sci Rep. 2023. PMID: 37188744 Free PMC article.
-
Synthesis and tyrosinase inhibitory activities of novel isopropylquinazolinones.BMC Chem. 2023 Jun 23;17(1):65. doi: 10.1186/s13065-023-00978-3. BMC Chem. 2023. PMID: 37353836 Free PMC article.
-
Synthesis and structure-activity relationship studies of benzimidazole-thioquinoline derivatives as α-glucosidase inhibitors.Sci Rep. 2023 Mar 16;13(1):4392. doi: 10.1038/s41598-023-31080-2. Sci Rep. 2023. PMID: 36928433 Free PMC article.
References
-
- Mahmoodi NO, Ghanbari Pirbasti F, Jalalifard Z. Recent advances in the synthesis of biscoumarin derivatives. J Chin Chem Soc. 2018;65(4):383–394.
-
- Carotti A, Carrieri A, Chimichi S, Boccalini M, Cosimelli B, Gnerre C, et al. Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorg Med Chem Lett. 2002;12(24):3551–3555. - PubMed
-
- Riaz M, Malik A. Daphsaifnin, a dimeric coumairn glucoside from Daphne oleoides. Heterocycles. 2001;55(4):769–773.
-
- Riaz M, Malik A. Structure determination of daphjamilin, a new bicoumarin glycoside, by NMR spectroscopy. Magn Reson Chem. 2001;39(10):641–642.
-
- Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem. 2005;12(8):887–916. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
