Malaria parasite heme biosynthesis promotes and griseofulvin protects against cerebral malaria in mice
- PMID: 35821013
- PMCID: PMC9276668
- DOI: 10.1038/s41467-022-31431-z
Malaria parasite heme biosynthesis promotes and griseofulvin protects against cerebral malaria in mice
Erratum in
-
Author Correction: Malaria parasite heme biosynthesis promotes and griseofulvin protects against cerebral malaria in mice.Nat Commun. 2024 Jun 25;15(1):5363. doi: 10.1038/s41467-024-49627-w. Nat Commun. 2024. PMID: 38918368 Free PMC article. No abstract available.
Abstract
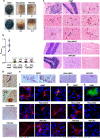
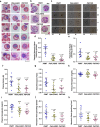
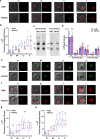
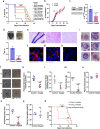
Heme-biosynthetic pathway of malaria parasite is dispensable for asexual stages, but essential for mosquito and liver stages. Despite having backup mechanisms to acquire hemoglobin-heme, pathway intermediates and/or enzymes from the host, asexual parasites express heme pathway enzymes and synthesize heme. Here we show heme synthesized in asexual stages promotes cerebral pathogenesis by enhancing hemozoin formation. Hemozoin is a parasite molecule associated with inflammation, aberrant host-immune responses, disease severity and cerebral pathogenesis. The heme pathway knockout parasites synthesize less hemozoin, and mice infected with knockout parasites are protected from cerebral malaria and death due to anemia is delayed. Biosynthetic heme regulates food vacuole integrity and the food vacuoles from knockout parasites are compromised in pH, lipid unsaturation and proteins, essential for hemozoin formation. Targeting parasite heme synthesis by griseofulvin-a FDA-approved antifungal drug, prevents cerebral malaria in mice and provides an adjunct therapeutic option for cerebral and severe malaria.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Significance of Plasmodium berghei Amino Acid Transporter 1 in Food Vacuole Functionality and Its Association with Cerebral Pathogenesis.Microbiol Spectr. 2023 Mar 28;11(2):e0494322. doi: 10.1128/spectrum.04943-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36976018 Free PMC article.
-
Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection.PLoS Pathog. 2013;9(8):e1003522. doi: 10.1371/journal.ppat.1003522. Epub 2013 Aug 1. PLoS Pathog. 2013. PMID: 23935500 Free PMC article.
-
Bazedoxifene, a Postmenopausal Drug, Acts as an Antimalarial and Inhibits Hemozoin Formation.Microbiol Spectr. 2022 Jun 29;10(3):e0278121. doi: 10.1128/spectrum.02781-21. Epub 2022 May 26. Microbiol Spectr. 2022. PMID: 35616371 Free PMC article.
-
Heme Detoxification in the Malaria Parasite: A Target for Antimalarial Drug Development.Acc Chem Res. 2021 Jun 1;54(11):2649-2659. doi: 10.1021/acs.accounts.1c00154. Epub 2021 May 13. Acc Chem Res. 2021. PMID: 33982570 Free PMC article. Review.
-
Plasmodium's bottomless pit: properties and functions of the malaria parasite's digestive vacuole.Trends Parasitol. 2022 Jul;38(7):525-543. doi: 10.1016/j.pt.2022.02.010. Epub 2022 Mar 19. Trends Parasitol. 2022. PMID: 35317985 Review.
Cited by
-
Plasmodium berghei HMGB1 controls the host immune responses and splenic clearance by regulating the expression of pir genes.J Biol Chem. 2024 Nov;300(11):107829. doi: 10.1016/j.jbc.2024.107829. Epub 2024 Sep 27. J Biol Chem. 2024. PMID: 39341498 Free PMC article.
-
Heme metabolism mediates RANKL-induced osteoclastogenesis via mitochondrial oxidative phosphorylation.J Bone Miner Res. 2025 May 24;40(5):639-655. doi: 10.1093/jbmr/zjaf040. J Bone Miner Res. 2025. PMID: 40073838 Free PMC article.
-
Distinct evolution of type I glutamine synthetase in Plasmodium and its species-specific requirement.Nat Commun. 2023 Jul 14;14(1):4216. doi: 10.1038/s41467-023-39670-4. Nat Commun. 2023. PMID: 37452051 Free PMC article.
-
Effect of neem leaf extract (Azadirachta indica) in reducing the degree of parasitemia and apoptosis in C57BL mice with cerebral malaria.Vet World. 2024 Jul;17(7):1497-1503. doi: 10.14202/vetworld.2024.1497-1503. Epub 2024 Jul 10. Vet World. 2024. PMID: 39185035 Free PMC article.
-
Antitumor properties of griseofulvin and its toxicity.Front Pharmacol. 2024 Sep 3;15:1459539. doi: 10.3389/fphar.2024.1459539. eCollection 2024. Front Pharmacol. 2024. PMID: 39314753 Free PMC article. Review.
References
-
- World Health Organization. World Malaria Reporthttps://www.who.int/teams/global-malaria-programme/reports/world-malaria... (2021).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

