Mycobacterium intracellulare induces a Th17 immune response via M1-like macrophage polarization in canine peripheral blood mononuclear cells
- PMID: 35821058
- PMCID: PMC9276657
- DOI: 10.1038/s41598-022-16117-2
Mycobacterium intracellulare induces a Th17 immune response via M1-like macrophage polarization in canine peripheral blood mononuclear cells
Abstract
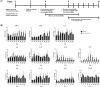
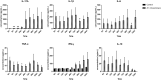
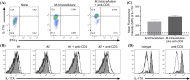
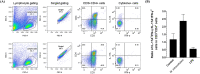
Mycobacterium avium-intracellulare complex (MAC) is one of the most prevalent pathogenic nontuberculous mycobacteria that cause chronic pulmonary disease. The prevalence of MAC infection has been rising globally in a wide range of hosts, including companion animals. MAC infection has been reported in dogs; however, little is known about interaction between MAC and dogs, especially in immune response. In this study, we investigated the host immune response driven by M. intracellulare using the co-culture system of canine T helper cells and autologous monocyte-derived macrophages (MDMs). Transcriptomic analysis revealed that canine MDMs differentiated into M1-like macrophages after M. intracellulare infection and the macrophages secreted molecules that induced Th1/Th17 cell polarization. Furthermore, canine lymphocytes co-cultured with M. intracellulare-infected macrophages induced the adaptive Th17 responses after 5 days. Taken together, our results indicate that M. intracellulare elicits a Th17 response through macrophage activation in this system. Those findings might help the understanding of the canine immune response to MAC infection and diminishing the potential zoonotic risk in One Health aspect.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
[Strategies for Mycobacterium avium complex infection control in Japan: how do they improve the present situation?].Kekkaku. 2013 Mar;88(3):355-71. Kekkaku. 2013. PMID: 23672176 Japanese.
-
Transcriptome analysis of long non-coding RNAs in Mycobacterium avium complex-infected macrophages.Front Immunol. 2024 Apr 22;15:1374437. doi: 10.3389/fimmu.2024.1374437. eCollection 2024. Front Immunol. 2024. PMID: 38711507 Free PMC article.
-
Mycobacterium avium Modulates the Protective Immune Response in Canine Peripheral Blood Mononuclear Cells.Front Cell Infect Microbiol. 2021 Jan 14;10:609712. doi: 10.3389/fcimb.2020.609712. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33520738 Free PMC article.
-
Modulating macrophage function to reinforce host innate resistance against Mycobacterium avium complex infection.Front Immunol. 2022 Nov 24;13:931876. doi: 10.3389/fimmu.2022.931876. eCollection 2022. Front Immunol. 2022. PMID: 36505429 Free PMC article. Review.
-
[M1 AND M2 MACROPHAGE POPULATIONS: THOSE INDUCED AND ACTIVATED BY MYCOBACTERIAL INFECTIONS].Kekkaku. 2016 Feb;91(2):75-82. Kekkaku. 2016. PMID: 27263230 Review. Japanese.
Cited by
-
Characterization of polarization states of canine monocyte derived macrophages.PLoS One. 2023 Nov 8;18(11):e0292757. doi: 10.1371/journal.pone.0292757. eCollection 2023. PLoS One. 2023. PMID: 37939066 Free PMC article.
-
Immunologic features of nontuberculous mycobacterial pulmonary disease based on spatially resolved whole transcriptomics.BMC Pulm Med. 2024 Aug 13;24(1):392. doi: 10.1186/s12890-024-03207-2. BMC Pulm Med. 2024. PMID: 39138424 Free PMC article.
-
Lymphocyte immunophenotype in dogs with immune-mediated hematologic disease.PLoS One. 2025 Jun 17;20(6):e0326341. doi: 10.1371/journal.pone.0326341. eCollection 2025. PLoS One. 2025. PMID: 40526723 Free PMC article.
References
-
- Malik R, et al. Ulcerated and nonulcerated nontuberculous cutaneous mycobacterial granulomas in cats and dogs. Vet. Dermatol. 2013;24:146–e133. - PubMed
-
- van Ingen J, et al. Proposal to elevate Mycobacterium avium complex ITS sequevar MAC-Q to Mycobacterium vulneris sp. nov. Int. J. Syst. Evol. Microbiol. 2009;59:2277–2282. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

