Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes
- PMID: 35821253
- PMCID: PMC9338301
- DOI: 10.1038/s41423-022-00894-x
Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes
Abstract
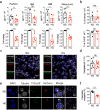
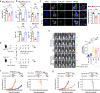
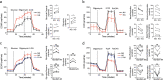
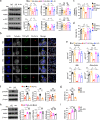
Patients with type 2 diabetes mellitus (T2DM) have an increased risk of cancer. The effect of glucose metabolism on γδ T cells and their impact on tumor surveillance remain unknown. Here, we showed that high glucose induced Warburg effect type of bioenergetic profile in Vγ9Vδ2 T cells, leading to excessive lactate accumulation, which further inhibited lytic granule secretion by impairing the trafficking of cytolytic machinery to the Vγ9Vδ2 T-cell-tumor synapse by suppressing AMPK activation and resulted in the loss of antitumor activity in vitro, in vivo and in patients. Strikingly, activating the AMPK pathway through glucose control or metformin treatment reversed the metabolic abnormalities and restored the antitumor activity of Vγ9Vδ2 T cells. These results suggest that the impaired antitumor activity of Vγ9Vδ2 T cells induced by dysregulated glucose metabolism may contribute to the increased cancer risk in T2DM patients and that metabolic reprogramming by targeting the AMPK pathway with metformin may improve tumor immunosurveillance.
Keywords: AMPK; Glucose metabolism; Lactate; T2DM; Tumor surveillance; γδ T cells.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

