Iron absorption and phosphate-lowering effects of ferric citrate hydrate are not influenced by gastric acid secretion inhibitors in patients with chronic kidney disease: a retrospective post hoc analysis
- PMID: 35821366
- PMCID: PMC9807476
- DOI: 10.1007/s11255-022-03287-1
Iron absorption and phosphate-lowering effects of ferric citrate hydrate are not influenced by gastric acid secretion inhibitors in patients with chronic kidney disease: a retrospective post hoc analysis
Abstract
Background: Ferric citrate hydrate (FC), an oral iron product is approved as iron preparation for iron deficiency anemia and phosphate binder for chronic kidney disease (CKD). We investigated whether gastric acid secretion inhibitors (GASI) influenced on iron absorption and phosphate-lowering effects of FC.
Methods: Two phase 3 studies of FC for treatment of hyperphosphatemia in CKD patients (non-dialysis-dependent, 12 weeks, and hemodialysis, 52 weeks), were retrospectively analyzed. Patients were divided into with or without concomitant GASI and levels of iron- and phosphate-related parameters were analyzed.
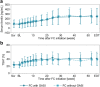
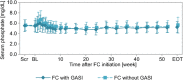
Results: In non-dialysis study (FC, 60 patients; placebo, 30 patients), 14 FC patients and 14 placebo patients used GASI. No significant differences were found between the FC and placebo groups for adjusted mean differences (95% CI) of changes from baseline to end of treatment (EOT) in serum ferritin [104.84 ng/mL (35.97, 173.71) with GASI vs 145.30 ng/mL (96.34, 194.25) without GASI, P = 0.34], and transferrin saturation (TSAT) [12.56% (- 0.83, 25.95) with GASI vs 18.56% (8.15, 28.98) without GASI, P = 0.49]. In hemodialysis study, 95/180 patients used GASI. Mean changes (SD) from baseline to EOT in serum ferritin were 166.32 ng/mL (153.70) with GASI and 155.16 ng/mL (139.47) without GASI, and for TSAT were 16.60% (19.44) with GASI and 16.02% (18.81) without GASI. In both studies, there were no differences in the changes from baseline to EOT in serum phosphate between with and without GASI cohorts.
Conclusion: GASI did not influence on the changes in serum ferritin, TSAT and serum phosphate by FC administration.
Keywords: Chronic kidney disease; Ferric citrate hydrate; Gastric acid secretion inhibitors; Histamine-2 receptor antagonist; Iron deficiency anemia; Proton pump inhibitor.
© 2022. The Author(s).
Conflict of interest statement
Kyoko Ito is an employee of Torii Pharmaceutical Co., Ltd. and Keitaro Yokoyama has received honoraria from Japan Tobacco Inc., Torii Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Kyowa Kirin Co., Ltd.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

