Phosphorus-Doped Graphene Aerogel as Self-Supported Electrocatalyst for CO2 -to-Ethanol Conversion
- PMID: 35821388
- PMCID: PMC9443446
- DOI: 10.1002/advs.202202006
Phosphorus-Doped Graphene Aerogel as Self-Supported Electrocatalyst for CO2 -to-Ethanol Conversion
Abstract
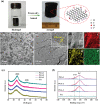
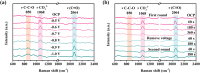
Electrochemical reduction of carbon dioxide (CO2 ) to ethanol is a promising strategy for global warming mitigation and resource utilization. However, due to the intricacy of C─C coupling and multiple proton-electron transfers, CO2 -to-ethanol conversion remains a great challenge with low activity and selectivity. Herein, it is reported a P-doped graphene aerogel as a self-supporting electrocatalyst for CO2 reduction to ethanol. High ethanol Faradaic efficiency (FE) of 48.7% and long stability of 70 h are achieved at -0.8 VRHE . Meanwhile, an outstanding ethanol yield of 14.62 µmol h-1 cm-2 can be obtained, outperforming most reported electrocatalysts. In situ Raman spectra indicate the important role of adsorbed *CO intermediates in CO2 -to-ethanol conversion. Furthermore, the possible active sites and optimal pathway for ethanol formation are revealed by density functional theory calculations. The graphene zigzag edges with P doping enhance the adsorption of *CO intermediate and increase the coverage of *CO on the catalyst surface, which facilitates the *CO dimerization and boosts the EtOH formation. In addition, the hierarchical pore structure of P-doped graphene aerogels exposes abundant active sites and facilitates mass/charge transfer. This work provides inventive insight into designing metal-free catalysts for liquid products from CO2 electroreduction.
Keywords: CO2 reduction; electrocatalysis; ethanol; graphene aerogel; phosphorus.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Li F., Li Y. C., Wang Z., Li J., Nam D.‐H., Lum Y., Luo M., Wang X., Ozden A., Hung S.‐F., Chen B., Wang Y., Wicks J., Xu Y., Li Y., Gabardo C. M., Dinh C.‐T., Wang Y., Zhuang T.‐T., Sinton D., Sargent E. H., Nat. Catal. 2020, 3, 75.
-
- Hu F., Yang L., Jiang Y., Duan C., Wang X., Zeng L., Lv X., Duan D., Liu Q., Kong T., Jiang J., Long R., Xiong Y., Angew. Chem., Int. Ed. 2021, 60, 26122. - PubMed
-
- Chen C., Yan X., Liu S., Wu Y., Wan Q., Sun X., Zhu Q., Liu H., Ma J., Zheng L., Wu H., Han B., Angew. Chem., Int. Ed. 2020, 59, 16459. - PubMed
-
- Lee S., Park G., Lee J., ACS Catal. 2017, 7, 8594.
-
- Wang H., Tzeng Y.‐K., Ji Y., Li J., Zheng X., Yang A., Liu Y., Gong Y., Cai L., Li Y., Zhang X., Chen W., Liu B., Lu H., Melosh N. A., Shen Z.‐X., Chan K., Tan T., Chu S., Cui Y., Nat. Nanotechnol. 2020, 15, 131. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
