The P2X1 receptor as a therapeutic target
- PMID: 35821454
- PMCID: PMC9832217
- DOI: 10.1007/s11302-022-09880-4
The P2X1 receptor as a therapeutic target
Abstract
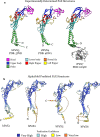
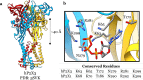
Within the family of purinergic receptors, the P2X1 receptor is a ligand-gated ion channel that plays a role in urogenital, immune and cardiovascular function. Specifically, the P2X1 receptor has been implicated in controlling smooth muscle contractions of the vas deferens and therefore has emerged as an exciting drug target for male contraception. In addition, the P2X1 receptor contributes to smooth muscle contractions of the bladder and is a target to treat bladder dysfunction. Finally, platelets and neutrophils have populations of P2X1 receptors that could be targeted for thrombosis and inflammatory conditions. Drugs that specifically target the P2X1 receptor have been challenging to develop, and only recently have small molecule antagonists of the P2X1 receptor been available. However, these ligands need further biological validation for appropriate selectivity and drug-like properties before they will be suitable for use in preclinical models of disease. Although the atomic structure of the P2X1 receptor has yet to be determined, the recent discovery of several other P2X receptor structures and improvements in the field of structural biology suggests that this is now a distinct possibility. Such efforts may significantly improve drug discovery efforts at the P2X1 receptor.
Keywords: ATP; Bladder dysfunction; Drug discovery; Male contraception; Structural biology; Thrombosis and inflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no conflict of interest.
Figures
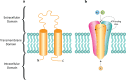

Similar articles
-
Smooth muscle does not have a common P2x receptor phenotype: expression, ontogeny and function of P2x1 receptors in mouse ileum, bladder and reproductive systems.Auton Neurosci. 2001 Sep 17;92(1-2):56-64. doi: 10.1016/S1566-0702(01)00319-8. Auton Neurosci. 2001. PMID: 11570704
-
P2X1 receptors localized in lipid rafts mediate ATP motor responses in the human vas deferens longitudinal muscles.Biol Reprod. 2014 Feb 6;90(2):23. doi: 10.1095/biolreprod.113.109660. Print 2014 Feb. Biol Reprod. 2014. PMID: 24352557
-
Nucleoside triphosphate diphosphohydrolase-1 ectonucleotidase is required for normal vas deferens contraction and male fertility through maintaining P2X1 receptor function.J Biol Chem. 2014 Oct 10;289(41):28629-39. doi: 10.1074/jbc.M114.604082. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25160621 Free PMC article.
-
P2X1: a unique platelet receptor with a key role in thromboinflammation.Platelets. 2021 Oct 3;32(7):902-908. doi: 10.1080/09537104.2021.1902972. Epub 2021 Mar 24. Platelets. 2021. PMID: 33760688 Review.
-
Calcium Signalling through Ligand-Gated Ion Channels such as P2X1 Receptors in the Platelet and other Non-Excitable Cells.Adv Exp Med Biol. 2016;898:305-29. doi: 10.1007/978-3-319-26974-0_13. Adv Exp Med Biol. 2016. PMID: 27161234 Review.
Cited by
-
Structural insights into the human P2X1 receptor and ligand interactions.Nat Commun. 2024 Sep 28;15(1):8418. doi: 10.1038/s41467-024-52776-7. Nat Commun. 2024. PMID: 39341830 Free PMC article.
-
A comprehensive review of the ten main platelet receptors involved in platelet activity and cardiovascular disease.Am J Blood Res. 2023 Dec 25;13(6):168-188. doi: 10.62347/NHUV4765. eCollection 2023. Am J Blood Res. 2023. PMID: 38223314 Free PMC article. Review.
-
The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling.Molecules. 2023 Feb 23;28(5):2102. doi: 10.3390/molecules28052102. Molecules. 2023. PMID: 36903347 Free PMC article. Review.
-
Untangling Macropore Formation and Current Facilitation in P2X7.Int J Mol Sci. 2023 Jun 30;24(13):10896. doi: 10.3390/ijms241310896. Int J Mol Sci. 2023. PMID: 37446075 Free PMC article. Review.
-
Structural basis of the multiple ligand binding mechanisms of the P2X1 receptor.Acta Pharmacol Sin. 2025 Sep;46(9):2564-2573. doi: 10.1038/s41401-025-01512-y. Epub 2025 Apr 2. Acta Pharmacol Sin. 2025. PMID: 40175702
References
-
- Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. In: International review of cytology. Academic Press, pp 31–304 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

