Real-World Effectiveness of Newly Initiated Systemic Therapy for Atopic Dermatitis in the United States: A Claims Database Analysis
- PMID: 35821555
- PMCID: PMC9402759
- DOI: 10.1007/s12325-022-02197-z
Real-World Effectiveness of Newly Initiated Systemic Therapy for Atopic Dermatitis in the United States: A Claims Database Analysis
Abstract
Introduction: Atopic dermatitis (AD) is associated with significant quality-of-life and economic burdens. Real-world evidence is needed to identify optimal treatment pathways for AD. Here we evaluate real-world effectiveness of systemic therapies for moderate-to-severe AD in the USA.
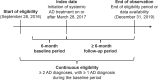
Methods: Data (September 2016 to December 2019) were from the IQVIA Health Plan Claims data set (IQVIA, Danbury, CT) from patients aged 12 years or older with AD (ICD-9/10-CM, 691.8/L20.x) initiating a systemic immunosuppressive (SIS) agent (methotrexate, cyclosporine, mycophenolate, or azathioprine) or dupilumab and continuously enrolled for at least 6 months before and after the index date. Indicators of non-response (i.e., adding on/switching systemic therapy, AD-related inpatient/emergency room visits, or incident staphylococcal/streptococcal skin infection) and predictors of non-response were evaluated. Descriptive statistics and Kaplan-Meier rates and times were obtained; Cox regression models were used.
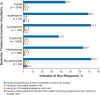
Results: In 3249 patients, 45.4% exhibited at least one indicator of non-response, with median time to non-response being longer for dupilumab than for any SIS therapy (27.0 vs 4.0-7.7 months, respectively). Key non-response predictors were age, geographic region, and baseline number of annual AD-related medical visits.
Conclusion: Non-response was common in patients with AD who required systemic treatment, and non-response indicators occurred significantly more frequently with SIS treatment than with dupilumab treatment.
Keywords: Atopic dermatitis; Dupilumab; Non-response; Real-world study; Systemic immunosuppressive agents.
© 2022. Pfizer Inc.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

