Targeting glycans for CAR therapy: The advent of sweet CARs
- PMID: 35821636
- PMCID: PMC9481985
- DOI: 10.1016/j.ymthe.2022.07.006
Targeting glycans for CAR therapy: The advent of sweet CARs
Abstract
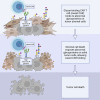
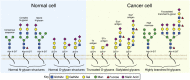
Chimeric antigen receptor (CAR) T cell therapy has created a paradigm shift in the treatment of hematologic malignancies but has not been as effective toward solid tumors. For such tumors, the primary obstacles facing CAR T cells are scarcity of tumor-specific antigens and the hostile and complex tumor microenvironment. Glycosylation, the process by which sugars are post-translationally added to proteins or lipids, is profoundly dysregulated in cancer. Abnormally glycosylated glycoproteins expressed on cancer cells offer unique targets for CAR T therapy as they are specific to tumor cells. Tumor stromal cells also express abnormal glycoproteins and thus also have the potential to be targeted by glycan-binding CAR T cells. This review will discuss the state of CAR T cells in the therapy of solid tumors, the cancer glycoproteome and its potential for use as a therapeutic target, and the landscape and future of glycan-binding CAR T cell therapy.
Keywords: CAR T cell therapy; cancer glycobiology; glycan; glycoprotein; solid tumor; tumor microenvironment.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

