Standard clinical approaches and emerging modalities for glioblastoma imaging
- PMID: 35821676
- PMCID: PMC9268747
- DOI: 10.1093/noajnl/vdac080
Standard clinical approaches and emerging modalities for glioblastoma imaging
Abstract
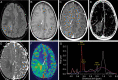
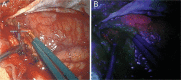
Glioblastoma (GBM) is the most common primary adult intracranial malignancy and carries a dismal prognosis despite an aggressive multimodal treatment regimen that consists of surgical resection, radiation, and adjuvant chemotherapy. Radiographic evaluation, largely informed by magnetic resonance imaging (MRI), is a critical component of initial diagnosis, surgical planning, and post-treatment monitoring. However, conventional MRI does not provide information regarding tumor microvasculature, necrosis, or neoangiogenesis. In addition, traditional MRI imaging can be further confounded by treatment-related effects such as pseudoprogression, radiation necrosis, and/or pseudoresponse(s) that preclude clinicians from making fully informed decisions when structuring a therapeutic approach. A myriad of novel imaging modalities have been developed to address these deficits. Herein, we provide a clinically oriented review of standard techniques for imaging GBM and highlight emerging technologies utilized in disease characterization and therapeutic development.
Keywords: MRI; PET; glioblastoma (GBM); mass spectrometry; radiographic progression; tumor progression.
© The Author(s) 2022. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures






References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
