Topical Anti-TNFα Agent Licaminlimab (OCS-02) Relieves Persistent Ocular Discomfort in Severe Dry Eye Disease: A Randomized Phase II Study
- PMID: 35821785
- PMCID: PMC9271287
- DOI: 10.2147/OPTH.S366836
Topical Anti-TNFα Agent Licaminlimab (OCS-02) Relieves Persistent Ocular Discomfort in Severe Dry Eye Disease: A Randomized Phase II Study
Abstract
Purpose: To assess the efficacy, safety, and pharmacokinetics of new topical ocular anti-TNFα antibody fragment licaminlimab in the relief of persistent ocular discomfort in severe dry eye disease (DED).
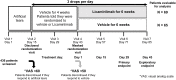
Patients and methods: Patients with ≥6-month history of DED, regular use of artificial tears, and best-corrected visual acuity (BCVA) of ≥55 letters in each eye (Early Treatment Diabetic Retinopathy Score) at baseline were included in this multicenter, randomized, vehicle-controlled, double masked study. A total of 514 patients were screened. After a 2-week run-in with Vehicle, all qualifying patients received Vehicle eye drops for 4 weeks. Patients with global ocular discomfort score ≥50 at the end of this 4-week period were randomized to receive licaminlimab (60 mg/mL ophthalmic solution) (69 patients) or Vehicle (65 patients) for 6 weeks. The primary efficacy endpoint was change from baseline in global ocular discomfort score at Day 29. Safety assessments included adverse events and ophthalmology examination including intraocular pressure (IOP). Serum licaminlimab levels were also determined.
Results: Change from baseline to Day 29 in global ocular discomfort score was statistically significantly greater for licaminlimab than for Vehicle (p = 0.041). No safety issues were identified. Serum licaminlimab was undetectable in most patients; the maximum concentration observed was 8.47 ng/mL.
Conclusion: Topical ocular licaminlimab demonstrated statistically significant improvement in global ocular discomfort score compared to Vehicle in patients with severe DED, with good tolerability, no increase in IOP, and minimal systemic drug exposure.
Keywords: anti-tumor necrosis factor α; dry eye disease; single-chain antibody fragment; topical treatment.
© 2022 Shettle et al.
Conflict of interest statement
J.W.S III and G.W were employees and equity holders of Alcon/Novartis at the time the study was conducted. L.S, J.M, and E.M received financial and non-financial support (grants) from Alcon Research Ltd for the conduct of the study. Outside of the reported study, L.S reports financial support from the following clinical research sponsors; Aerie Pharmaceuticals, Allysta Pharmaceutical Inc., Bausch & Lomb, Hovione Scientia Ltd, Kala Pharmaceuticals Inc., Novartis, Ocugen, Inc., Ocular Technologies Sarl, Ocular Therapeutix Inc., OmegaD LLC, Osmotica Pharmaceutical Corp., Santen Inc, Senju Pharmaceutical Co., Silk Technologies Ltd, Valeant Pharmaceuticals. Outside of the reported study, E.M reports financial support (grants) from the following; Aldeyra, Aurinia, Axero Vision, HanAll, Kowa, Mitotech, ReGenTree, Topivert. The authors report no other conflicts of interest in this work.
Figures
References
-
- Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S102–S106. - PubMed
Publication types
LinkOut - more resources
Full Text Sources