Combined Vitamin D, Omega-3 Fatty Acids, and a Simple Home Exercise Program May Reduce Cancer Risk Among Active Adults Aged 70 and Older: A Randomized Clinical Trial
- PMID: 35821820
- PMCID: PMC9261319
- DOI: 10.3389/fragi.2022.852643
Combined Vitamin D, Omega-3 Fatty Acids, and a Simple Home Exercise Program May Reduce Cancer Risk Among Active Adults Aged 70 and Older: A Randomized Clinical Trial
Abstract
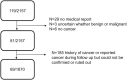
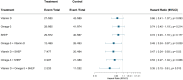
Objective: The aim of this study was to test the individual and combined benefit of vitamin D, omega-3, and a simple home strength exercise program on the risk of any invasive cancer. Design: The DO-HEALTH trial is a three-year, multicenter, 2 × 2 × 2 factorial design double-blind, randomized-controlled trial to test the individual and combined benefit of three public health interventions. Setting: The trial was conducted between December 2012 and December 2017 in five European countries. Participants: Generally healthy community-dwelling adults ≥70 years were recruited. Interventions: Supplemental 2000 IU/day of vitamin D3, and/or 1 g/day of marine omega-3s, and/or a simple home strength exercise (SHEP) programme compared to placebo and control exercise. Main outcome: In this pre-defined exploratory analysis, time-to-development of any verified invasive cancer was the primary outcome in an adjusted, intent-to-treat analysis. Results: In total, 2,157 participants (mean age 74.9 years; 61.7% women; 40.7% with 25-OH vitamin D below 20 /ml, 83% at least moderately physically active) were randomized. Over a median follow-up of 2.99 years, 81 invasive cancer cases were diagnosed and verified. For the three individual treatments, the adjusted hazard ratios (HRs, 95% CI, cases intervention versus control) were 0.76 (0.49-1.18; 36 vs. 45) for vitamin D3, 0.70 (0.44-1.09, 32 vs. 49) for omega-3s, and 0.74 (0.48-1.15, 35 vs. 46) for SHEP. For combinations of two treatments, adjusted HRs were 0.53 (0.28-1.00; 15 vs. 28 cases) for omega-3s plus vitamin D3; 0.56 (0.30-1.04; 11 vs. 21) for vitamin D3 plus SHEP; and 0.52 (0.28-0.97; 12 vs. 26 cases) for omega-3s plus SHEP. For all three treatments combined, the adjusted HR was 0.39 (0.18-0.85; 4 vs. 12 cases). Conclusion: Supplementation with daily high-dose vitamin D3 plus omega-3s, combined with SHEP, showed cumulative reduction in the cancer risk in generally healthy and active and largely vitamin D-replete adults ≥70 years. Clinical Trial Registration: ClinicalTrials.gov, Identifier: NCT01745263.
Keywords: cancer; co supplementation; combined treatment; exercise; healthy aging; omega-3; prevention; vitamin D.
Copyright © 2022 Bischoff-Ferrari, Willett, Manson, Dawson-Hughes, Manz, Theiler, Braendle, Vellas, Rizzoli, Kressig, Staehelin, Da Silva, Armbrecht, Egli, Kanis, Orav and Gaengler.
Conflict of interest statement
As part of the DO-HEALTH independent and investigator-initiated clinical trial, HB-F reports as the PI of the DO-HEALTH trial, grants from the European Commission (Grant Agreement No. 176;278588), from the University of Zurich, from NESTEC, from PFIZER Consumer Healthcare, from Streuli Pharma, plus non-financial support from DSM Nutritional Products and from Roche Diagnostics. Furthermore, HB-F reports speaker fees from Wild, Pfizer, Vifor, Mylan, Roche Diagnostics, and independent and investigator-initiated grants from Pfizer and from Vifor, outside the submitted work. JM reports grants from the National Institutes of Health, grants and non-financial support from Mars Symbioscience, outside the submitted work. BV reports personal fees from BIOGEN, CERECIN, ROCHE, and MSD, outside the submitted work. RR reports personal fees from Abiogen, Danone, Echolight, EMF, Mithra, ObsEva, Pfizer Consumer Health, and Theramex, outside the submitted work. JS is the scientific director of Forum D: a website dedicated to the critical revision and dissemination of knowledge regarding the medical uses of Vitamin D. EO reports a grant from Zurich University, during the conduct of the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bischoff-Ferrari H. A., De Godoi Rezende Costa Molino C., Rival S., Vellas B., Rizzoli R., Kressig R. W., et al. (2021). Do-HEALTH: Vitamin D3 - Omega-3 - Home Exercise - Healthy Aging and Longevity Trial - Design of a Multinational Clinical Trial on Healthy Aging Among European Seniors. Contemp. Clin. Trials 100, 106124. 10.1016/j.cct.2020.106124 - DOI - PubMed
-
- Bischoff-Ferrari H. A., Vellas B., Rizzoli R., Kressig R. W., Da Silva J. A. P., Blauth M., et al. (2020). Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults. JAMA 324, 1855–1868. 10.1001/jama.2020.16909 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

