Role of BAG5 in Protein Quality Control: Double-Edged Sword?
- PMID: 35821856
- PMCID: PMC9261338
- DOI: 10.3389/fragi.2022.844168
Role of BAG5 in Protein Quality Control: Double-Edged Sword?
Abstract
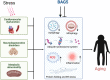
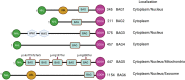
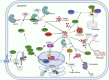
Cardiovascular disorder is the major health burden and cause of death among individuals worldwide. As the cardiomyocytes lack the ability for self-renewal, it is utmost necessary to surveil the protein quality in the cells. The Bcl-2 associated anthanogene protein (BAG) family and molecular chaperones (HSP70, HSP90) actively participate in maintaining cellular protein quality control (PQC) to limit cellular dysfunction in the cells. The BAG family contains a unique BAG domain which facilitates their interaction with the ATPase domain of the heat shock protein 70 (HSP70) to assist in protein folding. Among the BAG family members (BAG1-6), BAG5 protein is unique since it has five domains in tandem, and the binding of BD5 induces certain conformational changes in the nucleotide-binding domain (NBD) of HSP70 such that it loses its affinity for binding to ADP and results in enhanced protein refolding activity of HSP70. In this review, we shall describe the role of BAG5 in modulating mitophagy, endoplasmic stress, and cellular viability. Also, we have highlighted the interaction of BAG5 with other proteins, including PINK, DJ-1, CHIP, and their role in cellular PQC. Apart from this, we have described the role of BAG5 in cellular metabolism and aging.
Keywords: BAG5; Hsp70; aging; autophagy; cardiovascular; chaperone; mitophagy; neuroprotection.
Copyright © 2022 Gupta, Randhawa and Masternak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch.Int J Mol Sci. 2023 Dec 15;24(24):17531. doi: 10.3390/ijms242417531. Int J Mol Sci. 2023. PMID: 38139359 Free PMC article.
-
The C-terminal BAG domain of BAG5 induces conformational changes of the Hsp70 nucleotide-binding domain for ADP-ATP exchange.Structure. 2010 Mar 10;18(3):309-19. doi: 10.1016/j.str.2010.01.004. Structure. 2010. PMID: 20223214
-
Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate cancer and inhibits ER-stress induced apoptosis.BMC Cancer. 2013 Mar 1;13:96. doi: 10.1186/1471-2407-13-96. BMC Cancer. 2013. PMID: 23448667 Free PMC article.
-
The Link That Binds: The Linker of Hsp70 as a Helm of the Protein's Function.Biomolecules. 2019 Sep 27;9(10):543. doi: 10.3390/biom9100543. Biomolecules. 2019. PMID: 31569820 Free PMC article. Review.
-
BAG Family Members as Mitophagy Regulators in Mammals.Cells. 2022 Feb 15;11(4):681. doi: 10.3390/cells11040681. Cells. 2022. PMID: 35203329 Free PMC article. Review.
Cited by
-
Molecular Landscape of Tourette's Disorder.Int J Mol Sci. 2023 Jan 11;24(2):1428. doi: 10.3390/ijms24021428. Int J Mol Sci. 2023. PMID: 36674940 Free PMC article.
-
Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch.Int J Mol Sci. 2023 Dec 15;24(24):17531. doi: 10.3390/ijms242417531. Int J Mol Sci. 2023. PMID: 38139359 Free PMC article.
-
"Proteotranscriptomic analysis of advanced colorectal cancer patient derived organoids for drug sensitivity prediction".J Exp Clin Cancer Res. 2023 Jan 6;42(1):8. doi: 10.1186/s13046-022-02591-z. J Exp Clin Cancer Res. 2023. PMID: 36604765 Free PMC article.
-
A Bioinformatic Analysis of BAG Protein Interactors and Pathways in Alzheimer's and Parkinson's Disease.bioRxiv [Preprint]. 2025 Jul 23:2025.07.18.665492. doi: 10.1101/2025.07.18.665492. bioRxiv. 2025. PMID: 40777354 Free PMC article. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

