Examining the interactions of Galahad™ compound with viruses to develop a novel inactivated influenza A virus vaccine
- PMID: 35821966
- PMCID: PMC9258431
- DOI: 10.1016/j.heliyon.2022.e09887
Examining the interactions of Galahad™ compound with viruses to develop a novel inactivated influenza A virus vaccine
Abstract
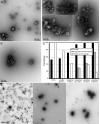
Galahad™ is a proanthocyanidin complexed with polysaccharides that inactivates viruses and indicates potential for an innovative approach to making protective vaccines. The polysaccharide portion of Galahad™ consists mainly of arabinan and arabinogalactan. In a seven-day toxicity study in rats, it was not toxic even when tested undiluted. Galahad™ inactivated a wide range of DNA and RNA viruses including adenoviruses, corona viruses such as SARS-CoV-2, and influenza viruses. Electron microscopy studies showed that exposure to Galahad™ caused extensive clumping of virions followed by lack of detection of virions after longer periods of exposure. Based on the viral inactivation data, the hypotheses tested is that Galahad™ inactivation of virus can be used to formulate a protective inactivated virus vaccine. To evaluate this hypothesis, infectious influenza A virus (H5N1, Duck/MN/1525/81) with a titer of 105.7 CCID50/0.1 ml was exposed for 10 min to Galahad™. This treatment caused the infectious virus titer to be reduced to below detectable limits. The Galahad™ -inactivated influenza preparation without adjuvant or preservative was given to BALB/c mice using a variety of routes of administration and dosing regimens. The most protective route of administration and dosing regimen was when mice were given the vaccine twice intranasally, the second dose coming 14 days after the primary vaccine dose. All the mice receiving this vaccine regimen survived the virus challenge while only 20% of the mice receiving placebo survived. This suggests that a Galahad™-inactivated influenza virus vaccine can elicit a protective immune response even without the use of an adjuvant. This technology should be investigated further for its potential to make effective human vaccines.
Keywords: Catechin; H5N1; Influenza; Polysaccharide; SARS-CoV-2 (COVID-19); Vaccine.
© 2022 The Author(s).
Conflict of interest statement
The authors declare the following conflict of interests: Thomas Konowalchuk owns shares of Galaxy Force Technologies, LLC that has patent rights to produce vaccines using GalahadTM. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Research on GalahadTM did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, but was financed by companies with ownership interest in GalahadTM without role in experimental design, data analysis, collection and interpretation.
Figures
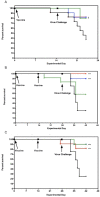
 105.7 TCID50∗P < 0.05.
105.7 TCID50∗P < 0.05.  104.7 TCID50 ∗P < 0.05.
104.7 TCID50 ∗P < 0.05.  103.7 TCID50 ∗P < 0.05.
103.7 TCID50 ∗P < 0.05.  No Vaccine (PSS). B. Vaccine 2: 10-minute exposure to Galahad™ with vaccine given twice intranasally to BALB/c mice and subsequently challenged with 103.5 TCID50 influenza A H5N1 (Duck/MN/1525/81).
No Vaccine (PSS). B. Vaccine 2: 10-minute exposure to Galahad™ with vaccine given twice intranasally to BALB/c mice and subsequently challenged with 103.5 TCID50 influenza A H5N1 (Duck/MN/1525/81).  105.7 TCID50 ∗∗∗P < 0.001.
105.7 TCID50 ∗∗∗P < 0.001.  104.7 TCID50 ∗∗∗P < 0.001.
104.7 TCID50 ∗∗∗P < 0.001.  103.7 TCID50..
103.7 TCID50..  No Vaccine (PSS). C. Vaccine 3 a 24-hour exposure to Galahad™ with vaccine given twice intranasally to BALB/c mice and subsequently challenged with 103.5 TCID50 influenza A H5N1 (Duck/MN/1525/81). Mice received vaccine 2 and 3 at 28 and 14 days before virus challenge; vaccine 1 14 days before virus challenge.
No Vaccine (PSS). C. Vaccine 3 a 24-hour exposure to Galahad™ with vaccine given twice intranasally to BALB/c mice and subsequently challenged with 103.5 TCID50 influenza A H5N1 (Duck/MN/1525/81). Mice received vaccine 2 and 3 at 28 and 14 days before virus challenge; vaccine 1 14 days before virus challenge.  105.7 TCID50 ∗∗∗P < 0.001.
105.7 TCID50 ∗∗∗P < 0.001.  104.7 TCID50 ∗∗∗P < 0.001.
104.7 TCID50 ∗∗∗P < 0.001.  103.7 TCID50.
103.7 TCID50.  No Vaccine (PSS).
No Vaccine (PSS).
 5.7 TCID50 ∗∗∗P < 0.001.
5.7 TCID50 ∗∗∗P < 0.001.  4.7 TCID50.
4.7 TCID50. 3.7 TCID50.
3.7 TCID50. No Vaccine (PSS).
No Vaccine (PSS).
 5.7 TCID50.
5.7 TCID50.  4.7 TCID50.
4.7 TCID50. 3.7 TCID50.
3.7 TCID50. No Vaccine (PSS).
No Vaccine (PSS).
 5.7 TCID50.
5.7 TCID50.  4.7 TCID50.
4.7 TCID50. 3.7 TCID50.
3.7 TCID50. No Vaccine (PSS).
No Vaccine (PSS).
 5.7 TCID50.
5.7 TCID50.  4.7 TCID50.
4.7 TCID50. 3.7 TCID50.
3.7 TCID50. No Vaccine (PSS).
No Vaccine (PSS).Similar articles
-
Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant.Virology. 2008 Oct 25;380(2):412-20. doi: 10.1016/j.virol.2008.08.002. Epub 2008 Sep 10. Virology. 2008. PMID: 18786689
-
Characterization of cross protection of Swine-Origin Influenza Virus (S-OIV) H1N1 and reassortant H5N1 influenza vaccine in BALB/c mice given a single-dose vaccination.J Biomed Sci. 2013 Mar 21;20(1):19. doi: 10.1186/1423-0127-20-19. J Biomed Sci. 2013. PMID: 23517052 Free PMC article.
-
An MDCK cell culture-derived formalin-inactivated influenza virus whole-virion vaccine from an influenza virus library confers cross-protective immunity by intranasal administration in mice.Clin Vaccine Immunol. 2013 Jul;20(7):998-1007. doi: 10.1128/CVI.00024-13. Epub 2013 May 1. Clin Vaccine Immunol. 2013. PMID: 23637045 Free PMC article.
-
Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus.BioDrugs. 2008;22(5):279-92. doi: 10.2165/00063030-200822050-00001. BioDrugs. 2008. PMID: 18778110 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
References
-
- Asbaghi O., Nazarian B., Reiner Ž., et al. The effects of grape seed extract on glycemic control, serum lipoproteins, inflammation, and body weight: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2020;34:239–253. - PubMed
-
- Fine A.M. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Alternative Med. Rev. 2000;5:144–151. PMID: 10767669. - PubMed
-
- Clouatre D.L., Kandaswami C., Connolly K.M. In: Encyclopedia of Dietary Supplements. second ed. Coates P.M., Betz J.M., Blackman M.R., et al., editors. Informa Healthcare; New York: 2010. Grape seed extract; pp. 391–401.
LinkOut - more resources
Full Text Sources
Miscellaneous

