Reduced Bordetella pertussis-specific CD4+ T-Cell Responses at Older Age
- PMID: 35822011
- PMCID: PMC9261443
- DOI: 10.3389/fragi.2021.737870
Reduced Bordetella pertussis-specific CD4+ T-Cell Responses at Older Age
Abstract
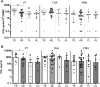
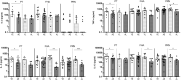
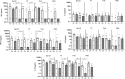
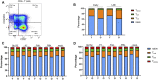
Pertussis, a human-specific respiratory infectious disease caused by the Gram-negative bacterium Bordetella pertussis (Bp), remains endemic with epidemic years despite high vaccination coverage. Whereas pertussis vaccines and natural infection with Bp confer immune protection, the duration of protection varies and is not lifelong. Recent evidence indicates a considerable underestimation of the pertussis burden among older adults. Whereas the impact of increasing age on Bp-specific humoral immunity has been demonstrated, little is known on immunosenescence of CD4+ T-cell responses in the context of Bp. Here, we aimed to address whether increasing age impacts responsiveness of the Bp-specific CD4+ T-cells in the memory pool following a clinically symptomatic pertussis infection in whole cell vaccine-primed pediatric and adult cases. Cytokine and proliferative responses and phenotypical profiles of CD4+ T cells specific for Bp antigens at an early and late convalescent timepoint were compared. Responses of various Th cytokines, including IFNγ, were significantly lower in older adults at early and late timepoints post diagnosis. In addition, we found lower frequencies of Bp-specific proliferated CD4+ T cells in older adults, in the absence of differences in replication profile. Phenotyping of Bp-specific CD4+ T cells suggested reduced expression of activation markers rather than increased expression of co-inhibitory markers. Altogether, our findings show that the magnitude and functionality of the Bp-specific memory CD4+ T-cell pool decrease at older age. Declined CD4+ T-cell responsiveness to Bp is suggested to contribute to the burden of pertussis in older adults.
Keywords: Bordetella pertussis; CD4+ T-cell cytokines; IFNγ ELISpot; aging; infection; proliferation.
Copyright © 2022 Lambert, van Twillert, Beckers, Poelen, Han, Pieren and van Els.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared consortium with one of the author CVE at time of review.
Figures

Similar articles
-
Lack of evidence supporting a role of IFN-β and TGF-β in differential polarization of Bordetella pertussis specific-T cell responses.Cytokine. 2021 Jan;137:155313. doi: 10.1016/j.cyto.2020.155313. Epub 2020 Sep 29. Cytokine. 2021. PMID: 33002739 Free PMC article.
-
Genome-wide characterization of T cell responses to Bordetella pertussis reveals broad reactivity and similar polarization irrespective of childhood vaccination profiles.bioRxiv [Preprint]. 2023 Mar 25:2023.03.24.534182. doi: 10.1101/2023.03.24.534182. bioRxiv. 2023. PMID: 36993748 Free PMC article. Preprint.
-
Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella Pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay.Vaccines (Basel). 2020 May 15;8(2):225. doi: 10.3390/vaccines8020225. Vaccines (Basel). 2020. PMID: 32429152 Free PMC article.
-
Superior B. pertussis Specific CD4+ T-Cell Immunity Imprinted by Natural Infection.Adv Exp Med Biol. 2019;1183:81-98. doi: 10.1007/5584_2019_405. Adv Exp Med Biol. 2019. PMID: 31321753 Review.
-
T-cell immune responses to Bordetella pertussis infection and vaccination.Pathog Dis. 2015 Oct;73(7):ftv051. doi: 10.1093/femspd/ftv051. Epub 2015 Aug 4. Pathog Dis. 2015. PMID: 26242279 Review.
Cited by
-
T cell reactivity to Bordetella pertussis is highly diverse regardless of childhood vaccination.Cell Host Microbe. 2023 Aug 9;31(8):1404-1416.e4. doi: 10.1016/j.chom.2023.06.015. Epub 2023 Jul 24. Cell Host Microbe. 2023. PMID: 37490913 Free PMC article.
-
Adaptive immune response to bordetella pertussis during vaccination and infection: emerging perspectives and unanswered questions.Expert Rev Vaccines. 2024 Jan-Dec;23(1):705-714. doi: 10.1080/14760584.2024.2383745. Epub 2024 Jul 24. Expert Rev Vaccines. 2024. PMID: 39037200 Free PMC article. Review.
References
-
- Bancroft T., Dillon M. B. C., da Silva Antunes R., Paul S., Peters B., Crotty S., et al. (2016). Th1 versus Th2 T Cell Polarization by Whole-Cell and Acellular Childhood Pertussis Vaccines Persists upon Re-immunization in Adolescence and Adulthood. Cell Immunol. 304-305, 35–43. 10.1016/j.cellimm.2016.05.002 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

