mTORC1 Crosstalk With Stress Granules in Aging and Age-Related Diseases
- PMID: 35822040
- PMCID: PMC9261333
- DOI: 10.3389/fragi.2021.761333
mTORC1 Crosstalk With Stress Granules in Aging and Age-Related Diseases
Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) kinase is a master regulator of metabolism and aging. A complex signaling network converges on mTORC1 and integrates growth factor, nutrient and stress signals. Aging is a dynamic process characterized by declining cellular survival, renewal, and fertility. Stressors elicited by aging hallmarks such as mitochondrial malfunction, loss of proteostasis, genomic instability and telomere shortening impinge on mTORC1 thereby contributing to age-related processes. Stress granules (SGs) constitute a cytoplasmic non-membranous compartment formed by RNA-protein aggregates, which control RNA metabolism, signaling, and survival under stress. Increasing evidence reveals complex crosstalk between the mTORC1 network and SGs. In this review, we cover stressors elicited by aging hallmarks that impinge on mTORC1 and SGs. We discuss their interplay, and we highlight possible links in the context of aging and age-related diseases.
Keywords: MTOR; aging hallmarks; amino acids; autophagy; cellular signaling; insulin; stress; stress granules (SGs).
Copyright © 2021 Cadena Sandoval, Heberle, Rehbein, Barile, Ramos Pittol and Thedieck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
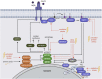
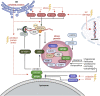
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

